A Single Atom of an Element: Identifying the Element with 21 Neutrons, 20 Electrons, and 20 Protons
Understanding the composition of atoms and the elements they belong to is a fundamental aspect of chemistry. In this article, we will explore a specific scenario where a single atom possesses 21 neutrons, 20 electrons, and 20 protons. By examining the properties of different elements, we will determine the specific element that fits this description.
Periodic Table of Elements
1. Atomic Structure:
To comprehend the nature of this scenario, we must first establish a brief understanding of atomic structure. Atoms are the building blocks of matter and consist of three primary subatomic particles: neutrons, electrons, and protons. Neutrons have no charge, electrons carry a negative charge, and protons possess a positive charge.
2. Neutrons, Electrons, and Protons in the Atom:
The given information states that the atom in question has 21 neutrons, 20 electrons, and 20 protons. Based on this data, we can analyze the possible elements that meet these criteria.
3. The Periodic Table:
The periodic table of elements provides a comprehensive framework for organizing and categorizing all known elements. By utilizing this valuable tool, we can narrow down our search for the element in question.
4. Comparing Neutron, Electron, and Proton Numbers:
To determine the specific element, we need to compare the given number of neutrons, electrons, and protons with the corresponding values for various elements. By examining the atomic numbers and atomic masses of elements, we can find potential matches.
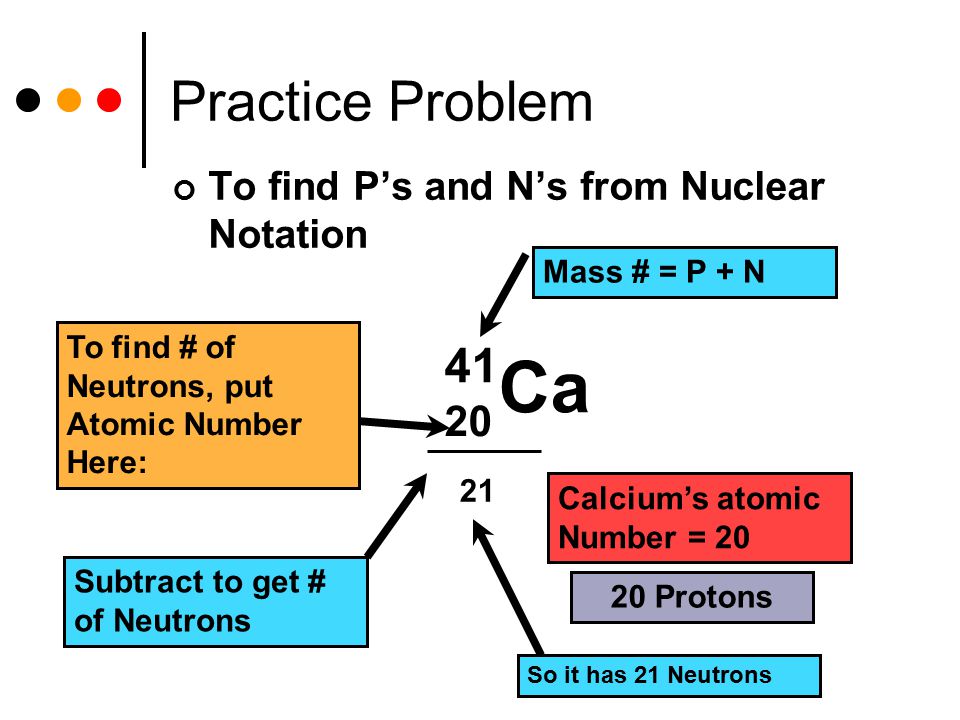
Atoms and The Periodic Table
5. Identifying the Element:
Based on the information provided, the element that has 21 neutrons, 20 electrons, and 20 protons is Calcium (Ca). Calcium is a chemical element with the atomic number 20, meaning it has 20 protons. The number of electrons in an atom is equal to the number of protons in a neutral atom, so Calcium also has 20 electrons. The atomic mass of Calcium is approximately 40, which means it has 20 neutrons.
In conclusion, a single atom with 21 neutrons, 20 electrons, and 20 protons corresponds to the element Calcium (Ca). By utilizing the periodic table and comparing the given data with the properties of various elements, we can confidently identify the specific element. Understanding the composition of atoms and their relationship to elements is crucial in the study of chemistry and forms the basis of many scientific applications.