Understanding the Ductility of Metals: Exploring the Reasons Behind their Flexibility
Metals are a vital class of materials known for their strength, durability, and various applications in industries ranging from construction to electronics. One intriguing property of metals is their ability to deform under stress without fracturing, known as ductility. In this article, we will delve into the reasons why metals are ductile instead of brittle, highlighting the structural and atomic factors that contribute to their flexibility.
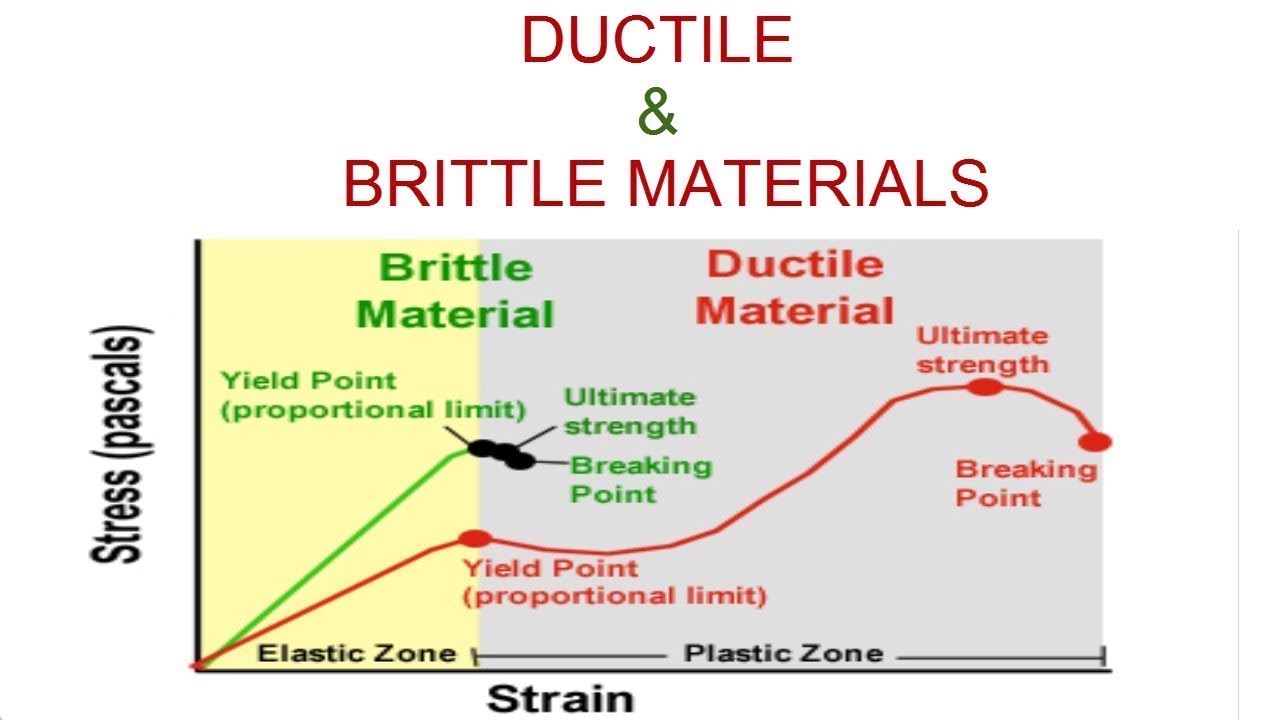
Ductile and Brittle materials
1. The Nature of Ductility and Brittleness
a. Ductility: Ductility refers to the ability of a material to undergo plastic deformation, allowing it to be drawn into wires or stretched without breaking. Ductile materials can withstand significant stress and strain before ultimate failure.
b. Brittleness: In contrast, brittle materials lack the ability to deform plastically and tend to fracture or break upon the application of stress. They exhibit limited flexibility and are more prone to sudden failure.
2. Atomic Arrangement and Bonding in Metals
a. Metallic Bonding: Metals possess a unique atomic arrangement and bonding known as metallic bonding. In this bonding type, metal atoms form a crystal lattice structure held together by a "sea" of delocalized electrons. This bonding allows for the movement of atoms and electrons within the metal structure.
b. Atomic Layers: Metals consist of closely packed atomic layers that can easily slide over one another. The presence of delocalized electrons between these layers enhances the mobility of atoms, facilitating plastic deformation under stress.
3. Slip Systems and Plastic Deformation
a. Slip Systems: Metals have specific crystallographic planes and directions known as slip systems. These planes and directions represent the paths of least resistance for atomic movement. When stress is applied, atoms in the crystal lattice can slide along these planes, leading to plastic deformation.
b. Dislocation Movement: Plastic deformation in metals occurs through the motion of dislocations. Dislocations are line defects within the crystal lattice where atomic planes are distorted. Under stress, dislocations move and propagate, allowing for the gradual rearrangement of atoms without causing immediate fracture.
4. Energy Absorption and Ductility
a. Energy Redistribution: Ductile materials have the ability to absorb and redistribute energy throughout their structure. When stress is applied, energy is dissipated and absorbed by the movement of dislocations and the reorganization of atomic bonds. This energy redistribution helps prevent localized stress concentrations and delays fracture.
b. Defect Tolerance: The presence of dislocations and the ability of atoms to move in metals make them more tolerant of defects and imperfections. Dislocations can accommodate strain and stress concentrations, minimizing the likelihood of catastrophic failure.
5. Alloying and Ductility
a. Alloy Formation: Alloying is the process of incorporating different elements into a metal to enhance its properties. The addition of alloying elements can significantly impact the ductility of metals.
b. Solid Solution Strengthening: Alloying can strengthen a metal by introducing solute atoms that impede the movement of dislocations. However, proper alloy design can balance strength and ductility, allowing for controlled plastic deformation without sacrificing overall mechanical performance.

Metal
The ductility of metals instead of brittleness can be attributed to several factors. The unique atomic arrangement and metallic bonding in metals enable the movement of atoms and the presence of dislocations, facilitating plastic deformation. The ability of metals to redistribute and absorb energy helps prevent immediate fracture and delays failure. Alloying further influences the ductility of metals by controlling the movement of dislocations and balancing strength and flexibility. Understanding these structural and atomic factors sheds light on why metals exhibit ductile behavior, making them essential materials in various industries.