Which Component is Affected When a Catalyst is Added to a Chemical Reaction?
Chemical reactions are fundamental processes that involve the transformation of reactants into products. These reactions often require certain conditions, such as specific temperatures and pressures, to proceed at a reasonable rate. However, the addition of a catalyst can significantly alter the reaction rate and outcome. In this article, we will explore the role of catalysts in chemical reactions and discuss which component is affected when a catalyst is added.
I. Understanding Catalysts:
1. Definition and Function: A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. It achieves this by providing an alternative reaction pathway with a lower activation energy, allowing the reaction to occur more easily. Catalysts enable reactions to proceed at milder conditions, reducing energy requirements and enhancing reaction efficiency.
2. Types of Catalysts: There are various types of catalysts, including heterogeneous catalysts, homogeneous catalysts, and enzyme catalysts. Heterogeneous catalysts exist in a different phase from the reactants, while homogeneous catalysts are in the same phase. Enzyme catalysts, on the other hand, are biological catalysts that facilitate reactions in living organisms.
Enzyme catalysts
II. The Effect of Catalysts on Reaction Components:
1. Reactants: When a catalyst is added to a chemical reaction, the reactants remain largely unaffected. The catalyst does not undergo any chemical changes and is not consumed during the reaction. Instead, it provides an alternative reaction pathway that lowers the activation energy for the reactants, making it easier for them to transform into products.
2. Activation Energy: The addition of a catalyst significantly affects the activation energy of a chemical reaction. Activation energy is the energy required for a reaction to occur and determines the rate at which the reaction proceeds. A catalyst lowers the activation energy by providing an alternative reaction pathway with fewer energy barriers, allowing the reaction to occur more readily.
3. Reaction Rate: The reaction rate is a crucial parameter affected by the addition of a catalyst. By reducing the activation energy, the catalyst increases the number of reactant molecules that have sufficient energy to overcome the energy barrier and proceed with the reaction. As a result, the reaction rate is enhanced, leading to faster product formation.
III. Catalysts and Equilibrium: Le Chatelier's Principle:
Catalysts do not affect the equilibrium constant or the position of an equilibrium in a chemical reaction. According to Le Chatelier's principle, catalysts facilitate both the forward and reverse reactions equally, thereby speeding up the establishment of equilibrium without shifting its position.
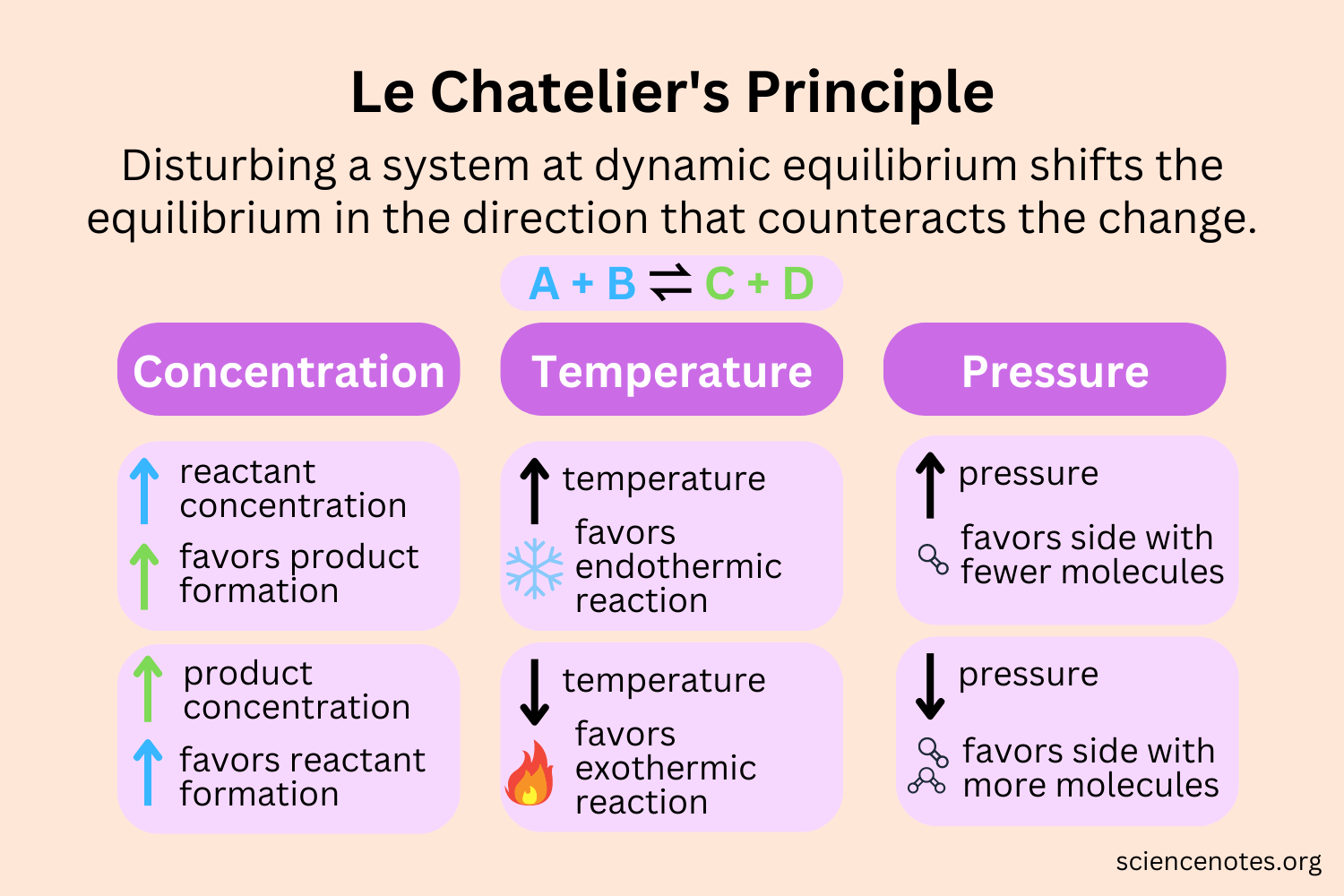
Le Chatelier's Principle
IV. Catalyst Poisoning and Deactivation:
1. Catalyst Poisoning: Certain substances, known as catalyst poisons, can inhibit the activity of catalysts. Catalyst poisons bind to the active sites of the catalyst, blocking its ability to facilitate the reaction. This interference can reduce the efficiency of the catalyst or render it completely inactive.
2. Catalyst Deactivation: Over time, catalysts can also undergo deactivation due to various factors, such as the accumulation of unwanted byproducts or the physical degradation of the catalyst material. Deactivation can result in reduced catalytic activity or the complete loss of activity, requiring the replacement or regeneration of the catalyst.
V. Conclusion:
In conclusion, catalysts play a vital role in chemical reactions by increasing the reaction rate without being consumed themselves. When a catalyst is added to a chemical reaction, the component primarily affected is the activation energy. By providing an alternative reaction pathway, catalysts lower the activation energy barrier and enhance the reaction rate. Reactants remain largely unaffected, and catalysts do not alter the equilibrium position.
However, catalysts can be poisoned or deactivated over time, diminishing their effectiveness. Understanding the impact of catalysts on reaction components is essential for optimizing reaction conditions and improving reaction efficiency.