Understanding the Orbital Notation of Carbon: A Fundamental Concept in Chemistry
Orbital notation plays a pivotal role in understanding the electronic structure of atoms and molecules, providing valuable insights into their chemical properties. In this article, we will focus on the orbital notation of carbon, a fundamental element in chemistry. We will explore how carbon's electrons are distributed in its atomic orbitals and discuss its significance in various chemical contexts.
1. The Basics of Orbital Notation
Orbital Notation
a. Orbitals and Electrons: An overview of atomic orbitals and the electrons they contain.
b. The Pauli Exclusion Principle: Understanding the principle that governs the distribution of electrons in orbitals.
2. The Carbon Atom
a. Carbon's Atomic Structure: An examination of carbon's atomic number, electron configuration, and orbital arrangement.
b. Importance of Carbon: Why carbon is considered the backbone of organic chemistry.
3. Electron Configuration of Carbon
a. Ground State Configuration: Describing the electron configuration of carbon in its ground state.
b. Notation Convention: Explaining the notation used to represent electron configurations.
4. Carbon's Orbital Notation
a. s and p Orbitals: Discussing the two types of orbitals involved in carbon's electron configuration.
b. Electron Filling: Demonstrating how electrons fill carbon's orbitals following the Aufbau principle.
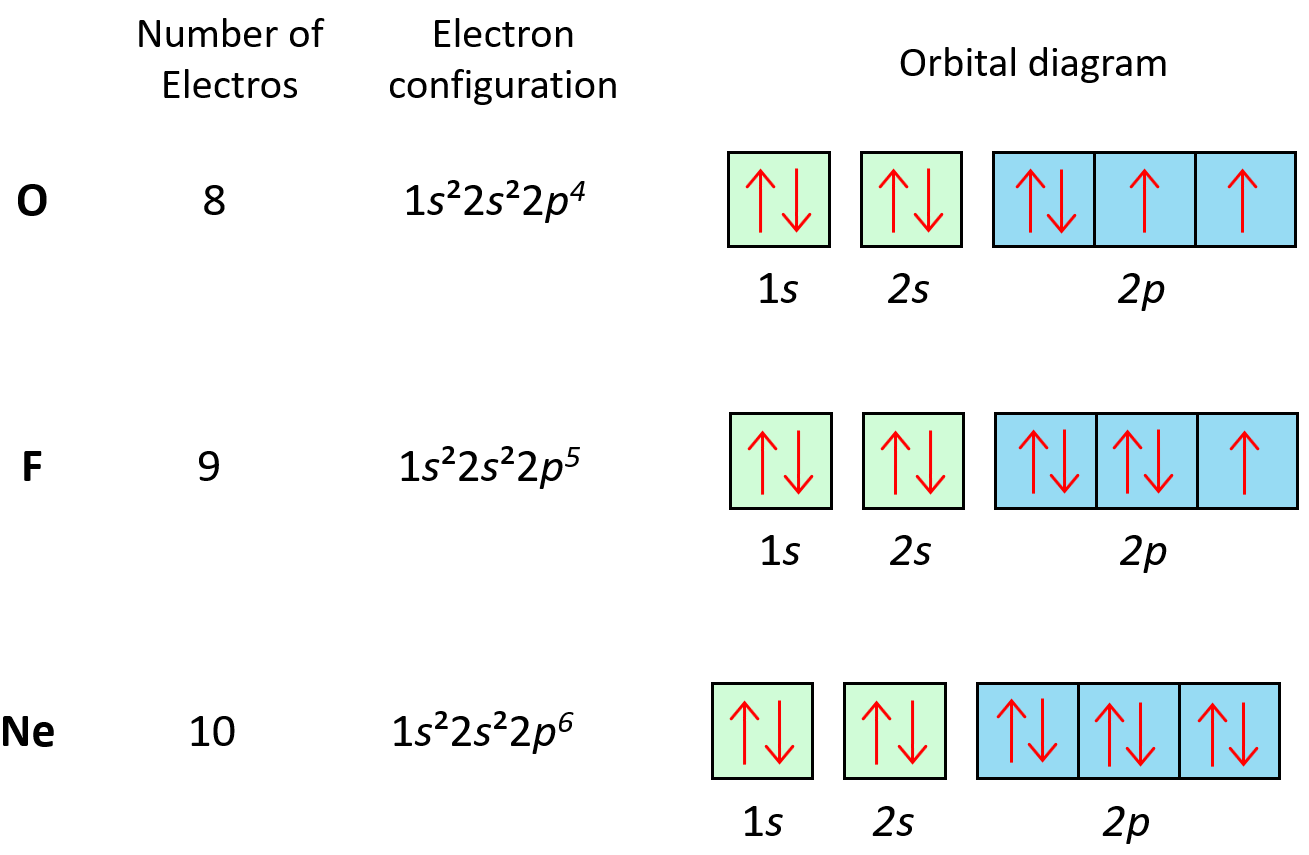
5. Orbital Diagram of Carbon
a. Visual Representation: Creating an orbital diagram to illustrate carbon's electron distribution.
b. Hund's Rule: Applying Hund's rule to determine electron spin orientations.
6. Chemical Implications
Chemical Implications
a. Carbon Bonding: How carbon's electron configuration influences its ability to form bonds.
b. Organic Chemistry: The connection between carbon's electronic structure and the diversity of organic compounds.
7. Variations in Carbon's Electron Configuration
a. Excited States: Exploring how carbon can exist in excited states with different electron configurations.
b. Chemical Reactivity: How these variations in electron configuration impact carbon's chemical reactivity.
8. Carbon Isotopes
a. Isotopic Variants: Briefly introducing carbon isotopes and their relevance.
b. Stable Carbon Isotopes: Carbon-12 and Carbon-13 and their importance in various fields.
9. Applications in Chemistry
a. Organic Synthesis: The role of carbon's electron configuration in designing and synthesizing organic compounds.
b. Materials Science: How carbon's properties contribute to the development of advanced materials.
In conclusion, understanding the orbital notation of carbon is a fundamental concept in chemistry that provides valuable insights into the behavior of this essential element. By delving into its electron configuration and orbital distribution, we gain a deeper appreciation of carbon's versatility and its central role in organic chemistry. Whether you're a student embarking on a chemistry journey or a curious mind interested in the building blocks of life, grasping carbon's orbital notation is a crucial step toward unlocking the secrets of the chemical world.