Understanding Chlorine Valence Electrons and Their Role in Chemistry
Chlorine is a chemical element that plays a crucial role in various chemical reactions and compounds. Understanding the concept of chlorine valence electrons is essential for comprehending its reactivity and its involvement in the formation of chemical bonds. In this comprehensive guide, we will delve into the world of chlorine valence electrons, exploring their significance, electron configuration, and their impact on chemical properties.
1. Chlorine Valence Electrons: An Overview

Chlorine Valence Electrons: An Overview
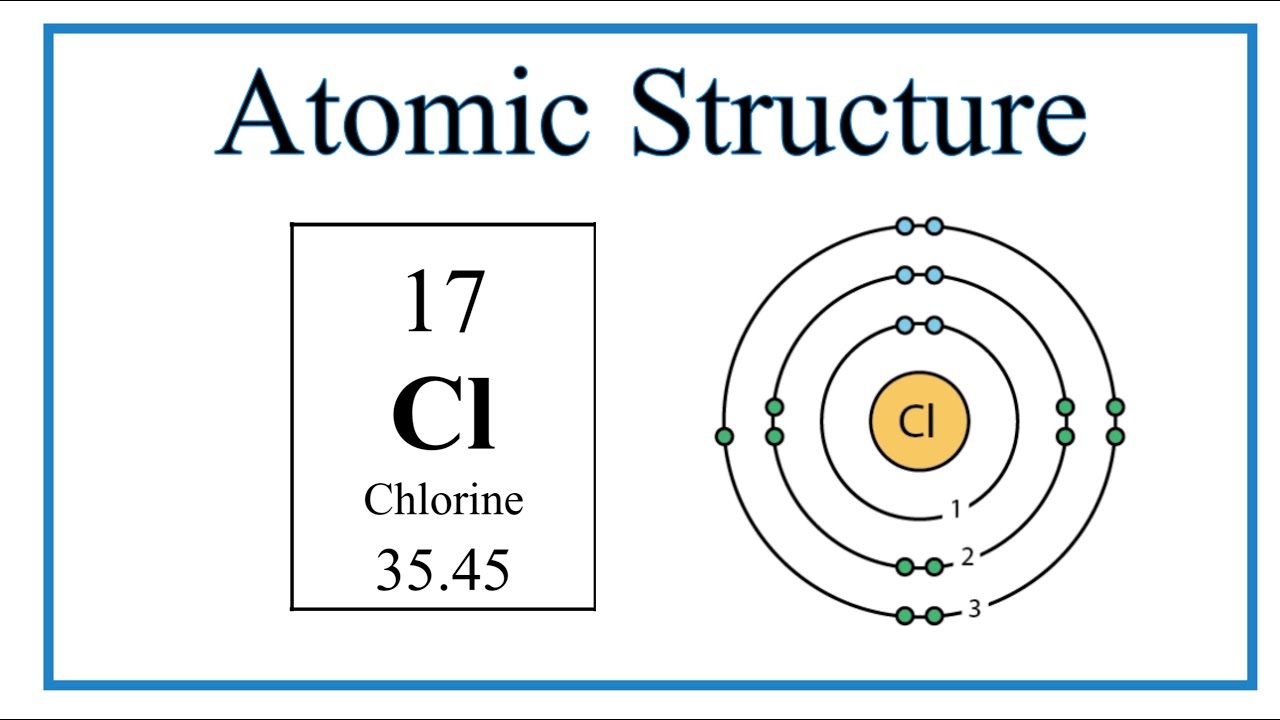
Chlorine (Cl) is a halogen element found in Group 17 of the periodic table. It possesses an atomic number of 17, indicating that it has 17 electrons. To understand its valence electrons, let's break down its electron configuration:
1s² 2s² 2pⶠ3s² 3pâµ
From this configuration, we can deduce that chlorine has 7 valence electrons in its outermost electron shell (3pâµ).
Comparing Chlorine Valence Electrons with Other Elements
To gain a deeper insight into the significance of chlorine valence electrons, let's compare them with valence electrons of other elements in Group 17, also known as the halogens:
2. The Role of Chlorine Valence Electrons in Chemical Bonding
The Role of Chlorine Valence Electrons in Chemical Bonding
Chlorine's reactivity is attributed to its 7 valence electrons, which strive to achieve a stable electron configuration similar to that of noble gases. To do so, chlorine readily gains an electron to achieve a full set of 8 electrons in its outer shell, forming the chloride ion (Clâ»).
Chlorine's electron affinity and electronegativity make it an essential element in various chemical compounds, including:
Sodium Chloride (NaCl): Common table salt, where chlorine forms an ionic bond with sodium.
Hydrochloric Acid (HCl): A strong acid where chlorine forms a covalent bond with hydrogen.
Chlorofluorocarbons (CFCs): Synthetic compounds used in refrigeration and aerosol propellants, containing chlorine atoms that impact ozone depletion.
Chlorine Gas (Clâ‚‚): A diatomic molecule formed when two chlorine atoms share electrons in a covalent bond.
3. Chlorine Valence Electrons in Biological Systems
Chlorine also plays a crucial role in biological systems. In the form of chloride ions (Clâ»), it is an essential electrolyte in the human body, involved in maintaining osmotic balance, nerve function, and muscle contraction.
In conclusion, understanding chlorine valence electrons is vital for comprehending the element's reactivity and its role in various chemical compounds. With 7 valence electrons in its outermost shell, chlorine exhibits a strong tendency to form chemical bonds, making it a versatile element in both chemical and biological systems. Its reactivity and electron configuration contribute to its significance in numerous applications, from table salt to industrial processes and biological functions.