The Valency of Phosphorus: Understanding Its Chemical Behavior
Phosphorus is a chemical element with a fascinating array of properties and applications. One of its fundamental characteristics is its valency, which plays a crucial role in its chemical behavior. In this article, we will delve into the valency of phosphorus, exploring its significance, variations, and implications in the world of chemistry.
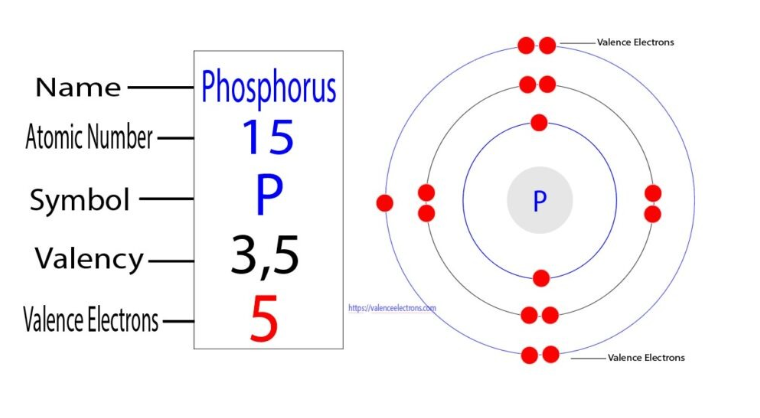
Phosphorus
1. What Is Valency in Chemistry?
Definition: Valency, in chemistry, is the measure of an element's ability to form chemical bonds. It represents the number of electrons an atom can gain, lose, or share to achieve a stable electron configuration.
Atomic Number: Phosphorus has an atomic number of 15, indicating it has 15 protons and 15 electrons.
Electron Configuration: Its electron configuration is 1s² 2s² 2pⶠ3s² 3p³, with three valence electrons in the 3p orbital.
2. Phosphorus Valency: An Overview
Common Valency: Phosphorus commonly exhibits a valency of 3 or 5.
Valency in Compounds: Its valency in compounds depends on the nature of the bonding and the surrounding elements.
3. Valency 3: Phosphorus as a Trivalent Element
Trivalent Compounds: In some compounds, phosphorus exhibits a valency of 3 by forming three covalent bonds.
Examples: Phosphorus trichloride (PCl₃) and phosphine (PH₃) are examples of compounds where phosphorus has a valency of 3.
4. Valency 5: Phosphorus as a Pentavalent Element
Pentavalent Compounds: In other compounds, phosphorus can have a valency of 5 by forming five covalent bonds.
Examples: Phosphorus pentachloride (PClâ‚…) and phosphorus pentoxide (Pâ‚„Oâ‚â‚€) illustrate phosphorus's pentavalent behavior.
5. Valency in Phosphates: An Important Example
Phosphates: In phosphate compounds, phosphorus typically exhibits a valency of 5, forming five covalent bonds with oxygen atoms.
Phosphate Importance: Phosphates are vital in biological systems, DNA structure, and cellular energy transfer (ATP).
6. Variability in Valency: Factors Influencing Phosphorus
Oxidation States: Phosphorus can exhibit a range of oxidation states, influencing its valency.
Electron Arrangement: The number of valence electrons and electron configuration impact the valency of phosphorus in various compounds.
7. Chemical Reactivity of Phosphorus
Phosphorus Variants: The different valencies of phosphorus contribute to its chemical reactivity and versatility in various chemical reactions.
Reduction and Oxidation: Phosphorus can undergo reduction and oxidation reactions, forming diverse compounds.
Fertilizers: Phosphate compounds are essential components of fertilizers, promoting plant growth.
Flame Retardants: Some phosphorus compounds are used as flame retardants in materials like textiles and plastics.
Chemical Industry: Phosphorus compounds play critical roles in the chemical industry, including the production of detergents and food additives.
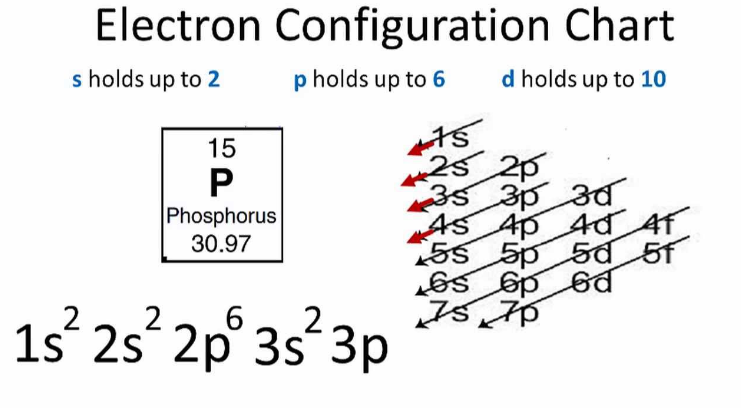
Electron COnfiguration chart
Versatility: The valency of phosphorus, whether trivalent or pentavalent, makes it a versatile and indispensable element in the realms of chemistry, biology, and industry.
Continuous Exploration: Researchers continue to explore the diverse applications and behaviors of phosphorus compounds, uncovering new possibilities for this essential element.
In conclusion, understanding the valency of phosphorus is essential for grasping its chemical behavior and significance in various applications. From fertilizers to flame retardants, phosphorus compounds continue to play a pivotal role in our daily lives and scientific advancements, showcasing the remarkable versatility of this element.