Unlocking the Chemistry of Ag + HCl: A Deep Dive into Silver Chloride Formation
The chemical reaction between silver (Ag) and hydrochloric acid (HCl) is a fundamental topic in chemistry, with important applications in various industries and scientific research. In this article, we will explore the intriguing world of Ag + HCl, delving into the reaction mechanism, its significance, and real-world applications.
1. Introduction to Ag + HCl Reaction
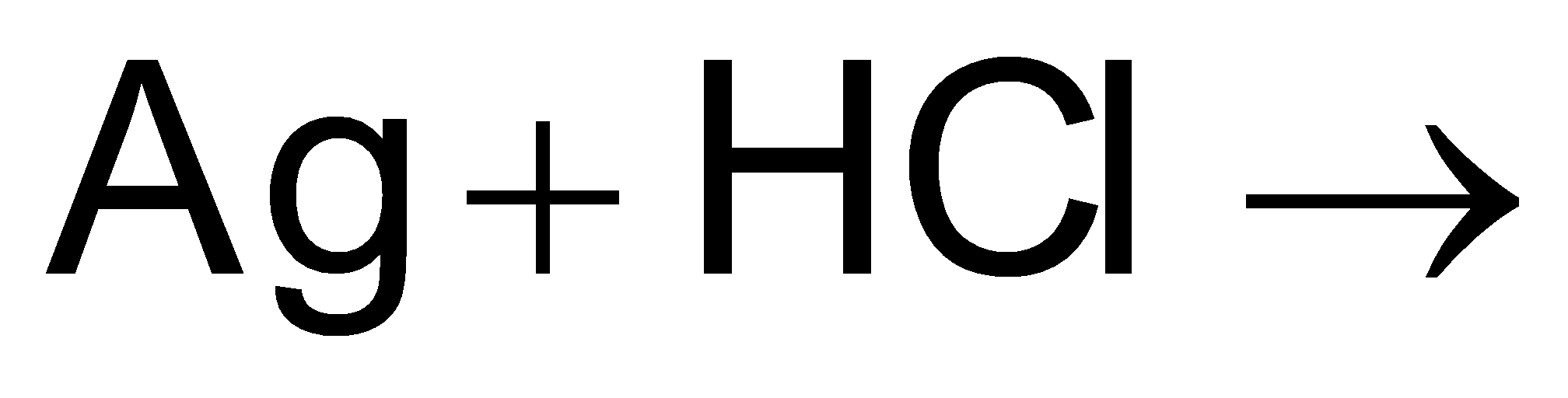
Ag + HCl Reaction
The Ag + HCl reaction represents the interaction between silver and hydrochloric acid, resulting in the formation of silver chloride (AgCl) and hydrogen gas (H2).
This chemical reaction is widely studied for its simplicity and the formation of a highly insoluble compound, silver chloride.
2. The Reaction Equation
The balanced chemical equation for the Ag + HCl reaction is as follows:
Ag + HCl → AgCl + H2
In this equation, a silver atom (Ag) reacts with a molecule of hydrochloric acid (HCl) to produce silver chloride (AgCl) and hydrogen gas (H2).
3. Reaction Mechanism
The Ag + HCl reaction involves the exchange of ions between the reactants. Specifically, the silver ion (Ag+) from the silver atom replaces the hydrogen ion (H+) in hydrochloric acid, resulting in the formation of silver chloride and the release of hydrogen gas.
This exchange of ions is the essence of the chemical reaction.
4. Significance of Ag + HCl Reaction
Ag + HCl Reaction
The Ag + HCl reaction holds great importance in chemistry and various fields:
Precipitation Reactions: The formation of silver chloride as an insoluble precipitate is utilized in analytical chemistry to detect the presence of chloride ions in solutions.
Photography: Silver chloride is a light-sensitive compound used in traditional black-and-white photography to capture images on photographic film.
Chemical Analysis: The reaction is used to quantitatively analyze chloride ions in various samples, such as water quality testing in environmental science.
Silver Extraction: In metallurgy, this reaction can be employed to extract silver from ores or silver-containing compounds.
Chemical Education: The Ag + HCl reaction serves as a foundational example in teaching chemistry principles, particularly in stoichiometry and balancing chemical equations.
5. Real-World Applications
The Ag + HCl reaction finds practical applications in several industries and scientific endeavors:
Water Treatment: The detection of chloride ions using silver chloride precipitation helps monitor water quality and ensure safe drinking water.
Photography: Although digital photography has largely replaced traditional methods, silver chloride is still used in some specialized photographic applications.
Mineral Processing: In the mining industry, the Ag + HCl reaction can be part of the process to extract valuable silver from ore.
Chemical Manufacturing: Some chemical processes may utilize this reaction as a part of a larger synthetic scheme.
6. Safety Considerations
While the Ag + HCl reaction is generally safe when conducted in controlled laboratory conditions, it's important to note that hydrochloric acid is corrosive and should be handled with care.
Proper safety precautions, including the use of appropriate personal protective equipment, should be observed when working with HCl.
In conclusion, the Ag + HCl reaction is a fundamental chemical process with significant implications in chemistry, analytical techniques, and various industries. Its role in the formation of silver chloride and the release of hydrogen gas makes it a valuable tool in chemical analysis, photography, water treatment, and more. Understanding the mechanism and applications of this reaction contributes to the broader knowledge of chemistry and its practical uses in the real world.