Understanding the Orbital Diagram for Chlorine
In the realm of chemistry, understanding the arrangement of electrons within an atom is crucial to comprehending its properties and behavior. One fundamental aspect of this understanding involves orbital diagrams, which depict the distribution of electrons in different energy levels and orbitals. This article delves into the intriguing world of orbital diagrams with a specific focus on chlorine, shedding light on its atomic structure and electronic configuration.
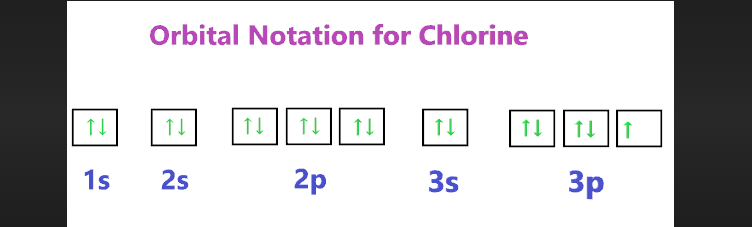
Orbital diagram for chlorine
1. Orbital Diagrams Unveiled:
An orbital diagram is a visual representation that showcases the distribution of electrons in an atom's orbitals.
These diagrams use arrows to indicate the direction of electron spin (up or down) and their respective energy levels.
By following a set of principles known as the Aufbau principle, Hund's rule, and the Pauli exclusion principle, scientists can accurately depict how electrons occupy different orbitals based on their energy.
2. Chlorine's Atomic Anatomy:
Chlorine, a halogen belonging to Group 17 (VIIA) of the periodic table, holds an atomic number of 17.
This number signifies the number of protons in its nucleus, which in turn equals the number of electrons.
In its ground state, chlorine's electron configuration is 1s² 2s² 2pⶠ3s² 3pâµ, highlighting the arrangement of electrons in various orbitals.
3. Breaking Down the Configuration:
Breaking down the electronic configuration, we find that the first energy level (n=1) contains 2 electrons in the 1s orbital.
The second energy level (n=2) accommodates 8 electrons - 2 in the 2s orbital and 6 in the 2p orbitals.
Finally, the third energy level (n=3) holds a total of 7 electrons - 2 in the 3s orbital and 5 in the 3p orbitals.
This completes chlorine's atomic structure with a total of 17 electrons.
4. Constructing the Orbital Diagram:
To construct the orbital diagram for chlorine, we start by representing each orbital with a box.
The boxes are organized by energy level, with the lowest energy level at the bottom.
Within each box, electrons are depicted as arrows pointing up or down, representing their spin. According to Hund's rule, electrons will first singly occupy each orbital in a subshell before pairing up.
5. Visualizing Chlorine's Orbital Diagram:
The 1s orbital is the first to be filled, with two arrows pointing in opposite directions to represent the two electrons.
Moving to the 2s orbital, another two electrons fill this space.
As we progress to the 2p orbitals, three individual electrons enter each of the three orbitals (Px, Py, and Pz) before any of them start pairing up.
This unpaired electron configuration in the 2p orbitals gives chlorine its reactivity and chemical behavior.
6. Significance in Chemistry:
Understanding chlorine's orbital diagram provides insights into its interactions and reactions.
The unpaired electrons in the 2p orbitals make chlorine highly reactive, seeking to gain an electron to achieve a stable electron configuration.
This reactivity is what makes chlorine a vital component in various chemical compounds and reactions, such as its role in forming ionic compounds with alkali metals.
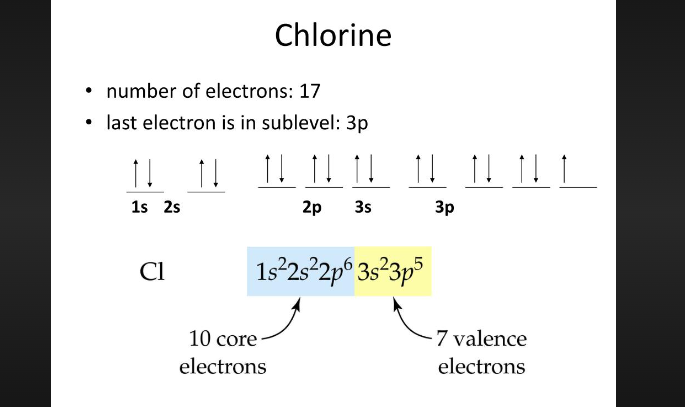
Information for chlorine
In the realm of atomic structure and chemistry, the orbital diagram for chlorine stands as a crucial tool for deciphering its behavior and properties. The arrangement of electrons within the atom's orbitals directly influences its reactivity and chemical interactions. By mastering the art of interpreting orbital diagrams, scientists can unlock the mysteries of elements and compounds, paving the way for advancements in various scientific and industrial applications.