Understanding the Number of Valence Electrons in Sodium (Na)
Valence electrons play a crucial role in determining an element's chemical properties and its ability to bond with other elements. Sodium (Na) is no exception. In this article, we will delve into the atomic structure of sodium and answer the question: "How many valence electrons does sodium have?" By understanding this fundamental aspect, we can gain insights into sodium's reactivity and its role in various chemical reactions.
1. The Structure of Sodium:
Before we explore the valence electrons of sodium, let's take a quick look at its atomic structure.
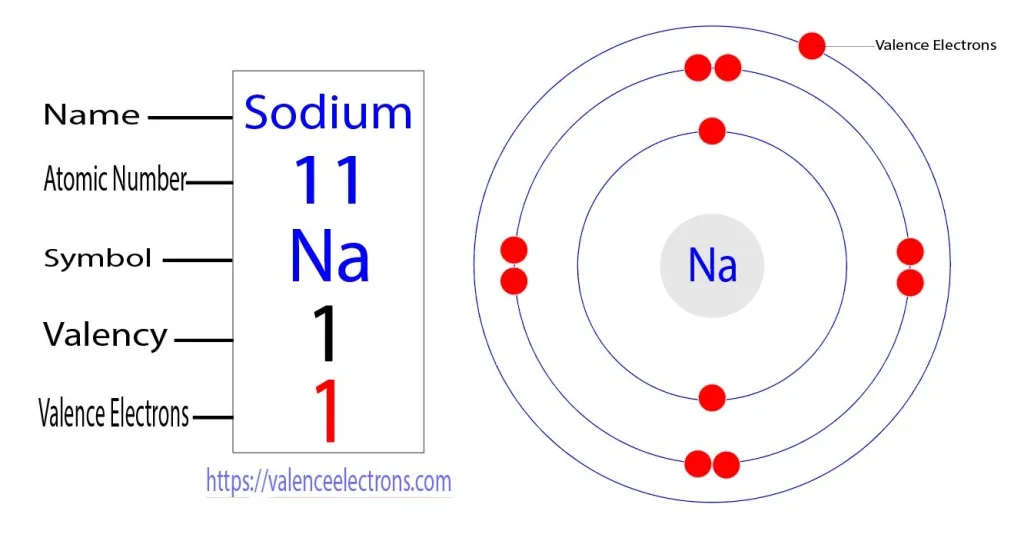
Sodium is an alkali metal with the atomic number 11, which indicates the presence of 11 protons in its nucleus.
The number of protons defines an element's identity. In its neutral state, sodium also has 11 electrons, arranged in energy levels or shells.
how many valence electrons does sodium (na) have?
2. Electron Configuration of Sodium:
To understand the distribution of electrons in sodium, we need to examine its electron configuration.
The electron configuration of an element provides a roadmap of how its electrons are distributed among different energy levels.
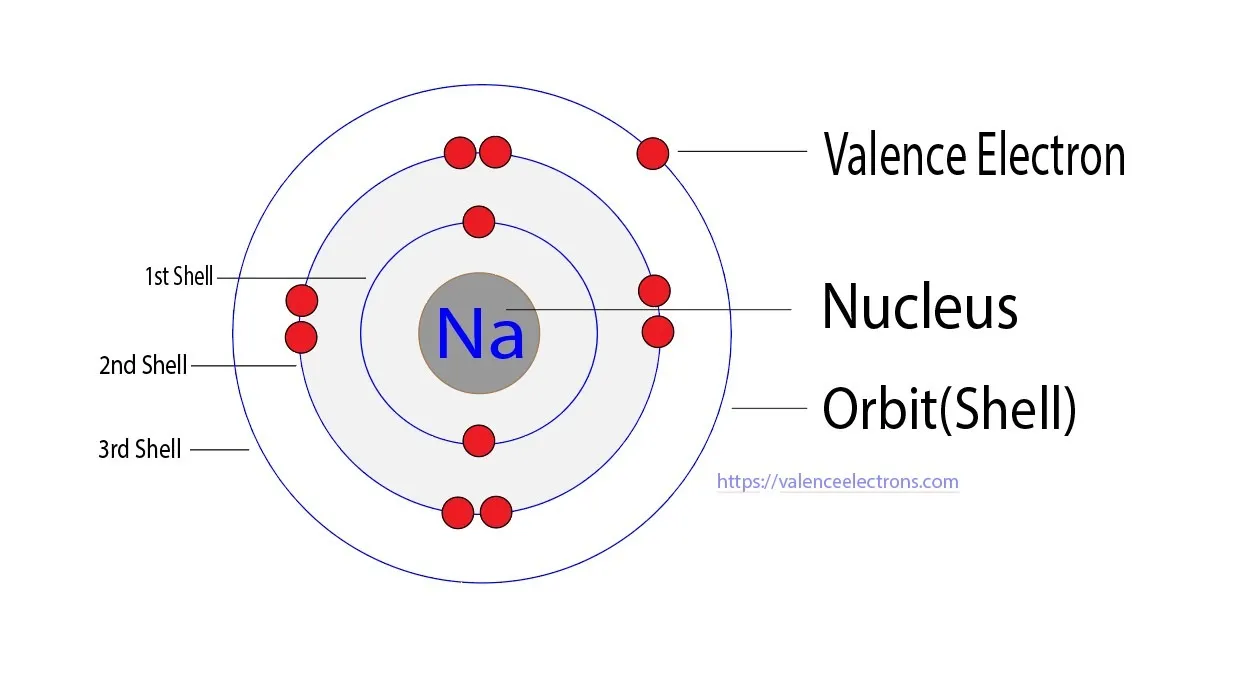
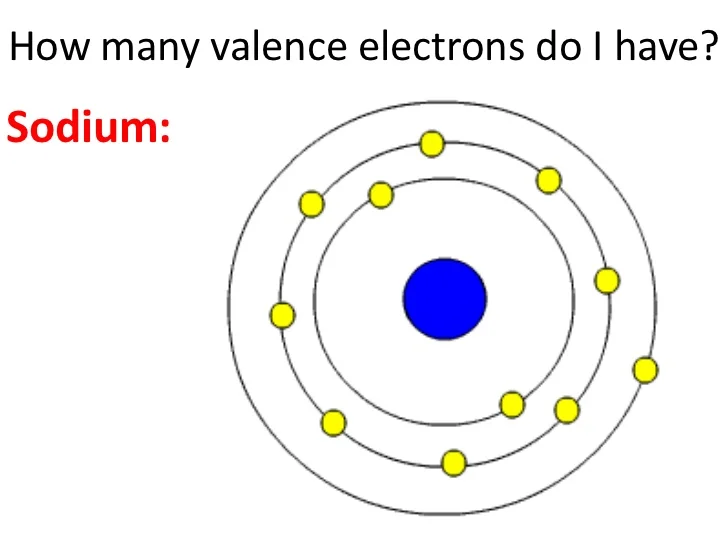
For sodium, the electron configuration is 1s² 2s² 2pⶠ3s¹.
This configuration signifies that sodium has two electrons in its first energy level, eight electrons in the second energy level, and one valence electron in the third energy level, specifically in the 3s orbital.
3. Valence Electrons and Reactivity:
Valence electrons are located in the outermost energy level of an atom and are crucial in determining how an element interacts with other elements.
In the case of sodium, its single valence electron in the 3s orbital is relatively far from the nucleus and is easily lost during chemical reactions.
This characteristic gives sodium its high reactivity. Sodium readily donates its valence electron to achieve a stable electron configuration similar to that of noble gases, which have full electron shells.
how many valence electrons does sodium (na) have?
4. Role in Ionic Bonding:
Sodium's tendency to lose its valence electron makes it a prime candidate for ionic bonding.
When sodium reacts with elements that require an additional electron to complete their valence shells, such as chlorine (Cl), sodium donates its valence electron to chlorine.
This results in the formation of positively charged sodium ions (Naâº) and negatively charged chloride ions (Clâ»).
The electrostatic attraction between these ions creates the iconic compound sodium chloride (NaCl), commonly known as table salt.
5. Practical Applications:
The knowledge of sodium's valence electron count is essential in various practical applications.
Sodium compounds are used extensively in industries, including the production of glass, detergents, and even in certain medical treatments.
Understanding sodium's reactivity helps engineers and scientists manipulate its properties to create specific desired effects in different contexts.
how many valence electrons does sodium (na) have?
6. Conclusion:
In conclusion, sodium possesses a single valence electron in its third energy level, making it highly reactive and prone to forming ionic bonds.
This unique characteristic has far-reaching implications in chemistry and industry.
By unraveling the mystery of sodium's valence electrons, we gain a deeper understanding of its behavior and its significance in our daily lives.
Whether it's the salt on our tables or the glass in our windows, sodium's valence electron plays a pivotal role.