Chromium Valence Electrons: Unveiling the Electron Configuration
In the world of chemistry, the arrangement of electrons in an atom's electron shells plays a pivotal role in determining its chemical behavior. Chromium, an essential transition metal, boasts a distinctive electron configuration that sets it apart. This article delves into the intricate details of chromium's valence electrons, shedding light on its electron arrangement, properties, and significance in various applications.
I. Introduction to Chromium and Its Significance
Chromium, a silvery-white transition metal denoted by the symbol Cr, holds immense importance in various industrial and technological domains. Its exceptional properties, such as corrosion resistance and the ability to form colorful compounds, have contributed to its widespread use. At the heart of these properties lies chromium's unique electron configuration, which governs its interactions with other elements.

Chromium
II. Electron Structure: Unraveling the Mystery
Chromium's electron configuration can be understood by delving into its atomic structure. With an atomic number of 24, chromium possesses 24 electrons. These electrons are distributed across different energy levels and orbitals. The most intriguing aspect of chromium's electron arrangement is its anomalous distribution in the 3d orbitals.
III. Chromium's Anomalous Electron Configuration
Unlike what might be expected, the electron configuration of chromium is not simply [Ar] 3dâ´ 4s². Instead, it takes on a unique form: [Ar] 3dâµ 4s¹. This seemingly counterintuitive configuration is a result of the interplay between energy levels and electron repulsions. The half-filled 3dâµ orbital configuration imparts stability to the atom, even though this arrangement requires some electrons to be paired up in the same orbital.
IV. Valence Electrons and Chemical Behavior
Chromium's valence electrons, which reside in the outermost energy level, have a significant impact on its chemical behavior. In this case, the valence electrons are the 4s¹ and the three unpaired electrons in the 3dâµ orbital. These unpaired electrons facilitate the formation of bonds with other elements, leading to the wide array of compounds that chromium can create.
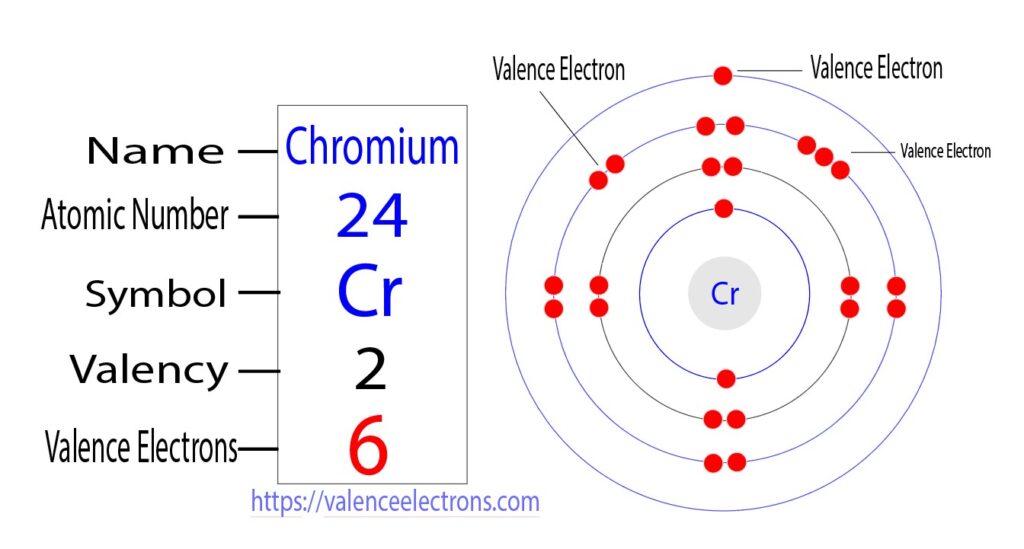
Valence Electrons of Chromium
V. Applications in Industry and Technology
Chromium's unique properties have found applications in various fields. Its resistance to corrosion has made it indispensable in the production of stainless steel, where it forms a passive oxide layer that shields the underlying metal. Additionally, its ability to produce vibrant colors when incorporated into compounds has made it a staple in the manufacturing of pigments and dyes.
VI. Beyond Earth: Chromium in Space
The study of elements extends beyond our planet. Chromium's presence in celestial bodies has been detected through spectroscopic analyses. Understanding its electron configuration provides insights into the conditions and processes that gave rise to these elements in the universe.
VII. Conclusion
Chromium's valence electrons, with their distinctive arrangement and influence on chemical reactivity, are a testament to the complexity and beauty of the atomic world. Its anomalous electron configuration, though seemingly perplexing, underlines the delicate balance between energy levels and electron repulsions. Whether it's enhancing the durability of everyday objects or coloring our world with vibrant hues, chromium's valence electrons continue to shape our lives in ways we might never have imagined.