Unveiling Magnesium's Valence Electron: A Key to Chemical Bonding
In the realm of chemistry, valence electrons play a crucial role in understanding the behavior and reactivity of elements. Magnesium, a widely studied alkaline earth metal, is no exception. If you're curious about magnesium's valence electron and its significance, you've come to the right place. This article explores the world of valence electrons, focusing on magnesium's unique characteristics and its impact on chemical bonding.
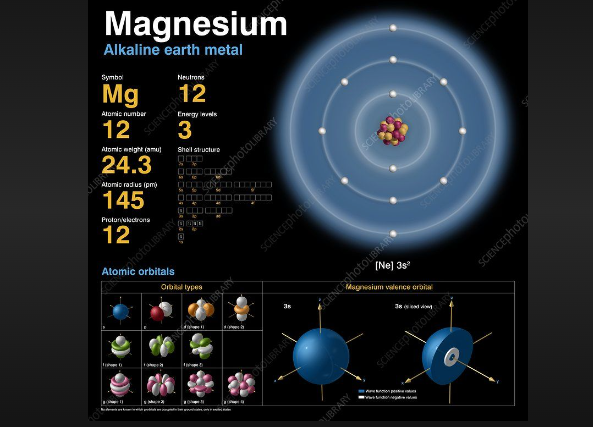
Valence electron of magnesium
1. Valence Electrons: The Foundation of Reactivity:
Before delving into magnesium's valence electron, let's establish a foundation by understanding the concept of valence electrons.
Valence electrons are the electrons found in the outermost energy level (electron shell) of an atom.
They determine an element's chemical properties, reactivity, and its ability to form chemical bonds.
2. Magnesium: A Glimpse into the Element:
Magnesium, with the atomic number 12, is an essential element found abundantly in the Earth's crust and within living organisms.
It's renowned for its role in biological processes and its presence in minerals like dolomite and magnesite.
3. Valence Electron of Magnesium:
Magnesium, situated in the second group of the periodic table, has two electron shells.
In its ground state, it has two electrons in the first energy level and eight electrons in the second energy level.
This configuration results in magnesium having two valence electrons, which are located in the outermost shell.
4. Significance of Two Valence Electrons:
The two valence electrons of magnesium play a crucial role in determining its chemical properties and reactivity.
With a goal to achieve a stable electron configuration, magnesium readily loses these two valence electrons to form a stable cation with a +2 charge.
5. Chemical Bonding and Reactivity:
Magnesium's tendency to lose its two valence electrons is the key to its reactivity and its ability to form chemical bonds with other elements.
This process is the foundation of magnesium's involvement in various chemical reactions and the formation of compounds.
6. Role in Ionic Bonding:
One of the most prominent examples of magnesium's valence electrons at work is its role in ionic bonding.
Magnesium readily loses its two valence electrons to achieve a stable electron configuration similar to that of noble gases.
As a result, it forms a +2 cation (Mg²âº) that can combine with negatively charged ions to create stable ionic compounds.
7. Applications and Significance:
Magnesium and its compounds have a wide range of applications due to its unique properties, facilitated by its valence electrons:
7.1. Alloy Production: Magnesium is often alloyed with other metals to enhance their properties, such as strength and corrosion resistance.
7.2. Medicine and Health: Magnesium compounds are used in medications and supplements to address magnesium deficiencies and support various bodily functions.
7.3. Pyrotechnics: Magnesium's bright white light emission when ignited makes it a popular choice for fireworks and flares.
7.4. Structural Applications: Magnesium alloys find use in aerospace, automotive, and engineering industries due to their lightweight and strong properties.
7.5. Metallurgy: Magnesium's ability to reduce oxides makes it useful in metallurgical processes for extracting metals from their ores.
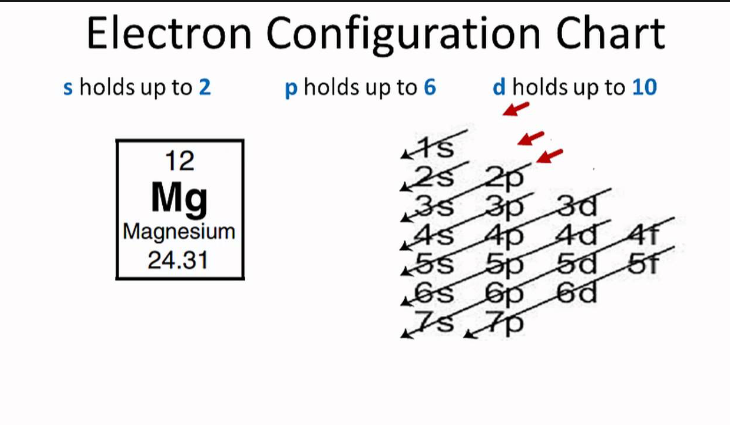
Electron configuration chart
Understanding magnesium's valence electron provides a window into its chemical behavior, reactivity, and its significant role in various applications. The two valence electrons in the outermost energy level are the driving force behind magnesium's participation in chemical reactions, ionic bonding, and compound formation. As we delve deeper into the world of chemistry, valence electrons like those in magnesium continue to unveil the mysteries of the elements and their interactions.