Unveiling the Valence Electrons of Zinc: Understanding Its Electron Configuration
Zinc, a versatile and essential element, plays a crucial role in various aspects of our lives, from industry to biology. The behavior of elements in chemical reactions is largely influenced by their electron configuration, with valence electrons being of particular significance. This article delves into the intriguing world of zinc's valence electrons, shedding light on their importance and how they impact the element's reactivity and chemical properties.
Section 1: The Basics of Valence Electrons
1.1 Electron Configuration:
Electrons, which orbit an atom's nucleus, are distributed in energy levels or shells.
The outermost shell, known as the valence shell, contains valence electrons that are involved in bonding and chemical reactions.
1.2 Valence Electrons' Role:
Valence electrons determine an element's chemical behavior, as they are the electrons available for forming bonds with other atoms.
valence electrons of zinc
Section 2: Zinc's Electron Configuration
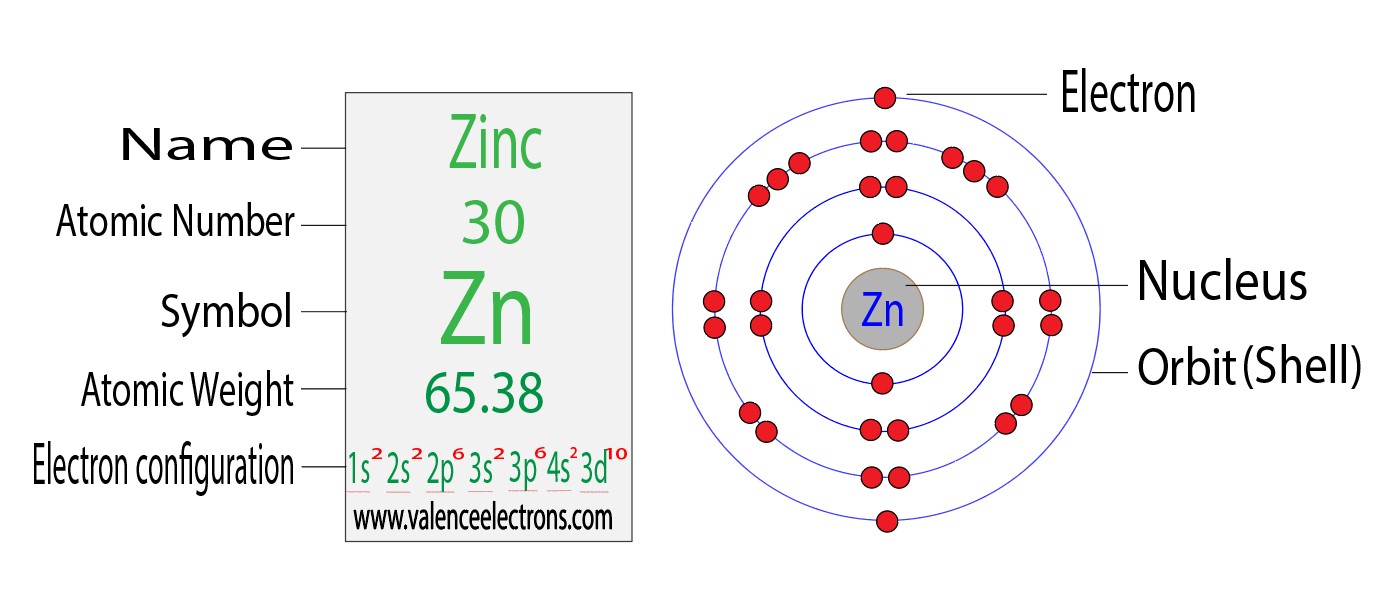
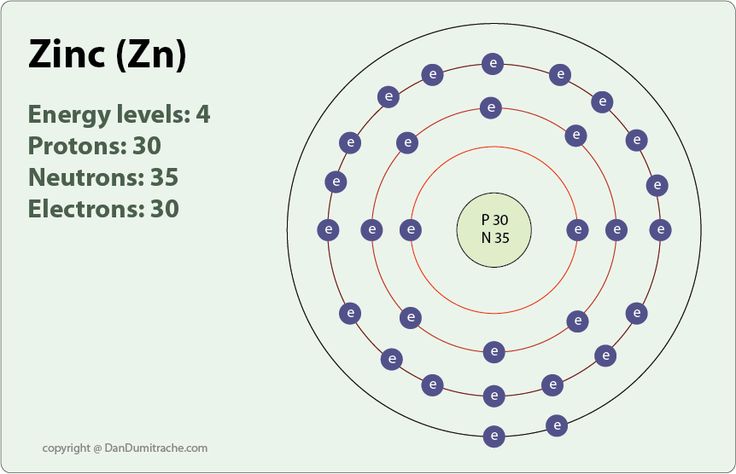
2.1 Atomic Number of Zinc:
Zinc, with an atomic number of 30, contains 30 electrons. These electrons are arranged in different energy levels around the nucleus.
2.2 Electron Arrangement:
Zinc's electron configuration is [Ar] 3d¹â° 4s², indicating that its inner electron shells are filled before its valence shell.
Section 3: Determining Valence Electrons in Zinc
3.1 Valence Electron Count:
Zinc's electron configuration suggests that its valence electrons are found in the 4s² orbital. Thus, zinc possesses 2 valence electrons.
3.2 Transition Metal Exception:
Zinc is classified as a transition metal, a group known for its unique electron configurations.
Despite being a d-block element, zinc's electron arrangement places its valence electrons in the s-orbital rather than the d-orbital.
valence electrons of zinc
Section 4: Impact of Valence Electrons on Zinc's Chemical Behavior
4.1 Reactivity:
The presence of 2 valence electrons in the 4s² orbital imparts specific reactivity to zinc. It has the potential to lose these 2 electrons and form a +2 oxidation state.
4.2 Bond Formation:
Zinc primarily forms bonds by losing its 2 valence electrons. This makes it a reducing agent in certain chemical reactions.
Section 5: Zinc's Applications and Importance
5.1 Industrial Applications:
Zinc's properties, including its reactivity due to valence electrons, make it valuable for various industrial processes. It is commonly used for galvanizing to protect metals from corrosion.
5.2 Biological Role:
Zinc is an essential trace element for living organisms. It is involved in enzyme function, DNA synthesis, and immune system support.
Section 6: Valence Electrons and Periodic Trends
6.1 Periodic Table Trends:
The periodic table exhibits trends in valence electrons across elements. Moving from left to right across a period, valence electrons increase, impacting reactivity.
6.2 Group Trends:
Elements within the same group have similar valence electron configurations, leading to analogous chemical behaviors. Zinc's position in Group 12 is unique due to its 4s² valence electrons.
Section 7: Practical Significance and Research
7.1 Chemical Research:
Understanding the valence electrons of zinc contributes to research in various fields, including materials science, catalysis, and environmental chemistry.
7.2 Educational Importance:
Knowledge of valence electrons enhances the understanding of chemistry students and researchers alike. It provides insights into predicting chemical reactions and behavior.
Zinc's valence electrons, specifically the 2 electrons in its 4s² orbital, play a fundamental role in its chemical behavior and properties. These electrons influence zinc's reactivity, its ability to form bonds, and its role in various industrial and biological processes. While the electron configuration of zinc deviates from traditional transition metal patterns, it underscores the importance of valence electrons in defining an element's chemistry. As we continue to explore the intricacies of elements and their electron arrangements, zinc's unique valence electron configuration serves as a fascinating subject of study and a key to unlocking its chemical secrets.