Understanding the Atomic Structure of Fluorine: Protons, Neutrons, and Electrons
The study of chemistry involves delving into the intricate details of atoms and their composition. Fluorine, a highly reactive chemical element, is no exception. In this article, we will explore the atomic structure of fluorine, including the number of protons, neutrons, and electrons it possesses.

How many protons neutrons and electrons does fluorine have?
1. Basic Atomic Structure:
Atoms are the building blocks of matter.
They consist of a nucleus, which contains protons and neutrons, surrounded by a cloud of electrons. The number of protons determines an element's identity, while the sum of protons and neutrons gives its mass number.
2. Fluorine's Atomic Number and Identity:
Fluorine is represented by the chemical symbol "F" on the periodic table.
Its atomic number is 9, indicating that it has 9 protons in its nucleus. This unique atomic number distinguishes fluorine from other elements.
3. Protons - The Identity Builders:
Protons are positively charged particles found in the nucleus of an atom
In the case of fluorine, the 9 protons it possesses are essential for identifying it as an element distinct from others.
4. Neutrons - Adding to the Mass:
Neutrons, as the name suggests, are electrically neutral particles found alongside protons in the nucleus.
The number of neutrons can vary within an element's different isotopes.
Fluorine usually has 10 neutrons, making its most common isotope fluorine-19.
5. Mass Number - The Sum of Protons and Neutrons:
The mass number is calculated by adding the number of protons and neutrons in an atom's nucleus.
For fluorine-19, this sum is 9 (protons) + 10 (neutrons) = 19. This is why it's called fluorine-19.
6. Electrons - The Cloud of Negativity:
Electrons are negatively charged particles that orbit the nucleus in energy levels or electron shells.
Fluorine, with 9 protons, also has 9 electrons to balance the positive charge of the protons.
7. Chemical Properties of Fluorine:
Fluorine's atomic structure directly influences its chemical behavior.
With 9 electrons, it has an electron configuration of 1s² 2s² 2pâµ, leaving it with only one vacant spot in its outermost shell.
This makes fluorine highly reactive, eager to gain one more electron to achieve a stable electron configuration.
8. Fluorine's Electron Affinity:
Fluorine's electron affinity, a measure of how much it wants to gain an electron, is the highest of all elements.
This intense desire to complete its electron shell makes fluorine a strong oxidizing agent and highly reactive in chemical reactions.
9. Practical Applications:
Fluorine and its compounds are widely used in various industries and applications.
Fluorine-based compounds are used in toothpaste to prevent tooth decay, and they play a crucial role in the production of semiconductor devices.
10. The Importance of Atomic Structure:
Understanding the atomic structure of elements like fluorine is fundamental to understanding their properties and behaviors.
It's a cornerstone of chemistry that underpins a range of scientific and practical applications.
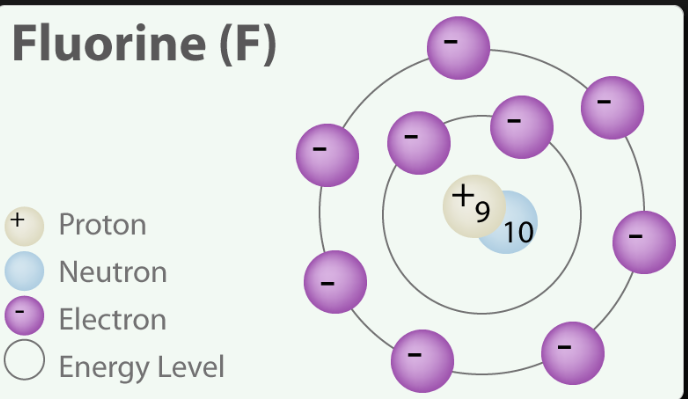
Fluorine have protons neutrons and electrons
Fluorine's atomic structure is composed of 9 protons, 10 neutrons, and 9 electrons. This arrangement gives fluorine its unique chemical properties, making it a highly reactive and sought-after element in various industries. The interplay of protons, neutrons, and electrons in atoms is the foundation of the world of chemistry, enabling us to understand and manipulate the substances around us.