Understanding Zinc Orbital Diagram: Unveiling the Electron Arrangement
The zinc orbital diagram is a visual representation of the electron arrangement within the zinc atom. In this article, we will explore the significance of the zinc orbital diagram, its role in understanding the electronic structure of zinc, and its relevance in the field of chemistry.
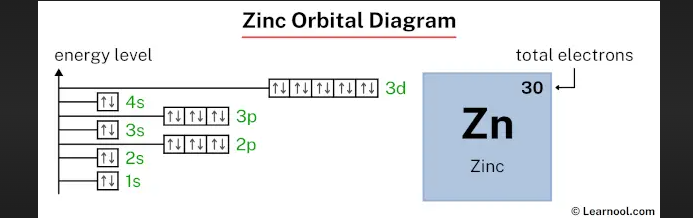
Zinc orbital diagram
Part 1: The Basics of Orbital Diagrams
1.1 What are Orbital Diagrams?
Orbital diagrams are graphical representations that illustrate the distribution of electrons in the atomic orbitals of an element. They provide a clear depiction of electron occupancy and help in understanding an atom's electronic configuration.
1.2 Importance in Chemistry:
Orbital diagrams are crucial for understanding an element's properties, chemical reactivity, and bonding behavior. They serve as a fundamental tool in explaining the behavior of atoms in various chemical processes.
Part 2: The Zinc Element
2.1 Introduction to Zinc:
Zinc is a chemical element with the symbol Zn and atomic number 30. It is a transition metal known for its bluish-white appearance and its vital role in various biological processes and industrial applications.
2.2 Electronic Configuration of Zinc:
The electron configuration of an element defines the arrangement of electrons in its atomic orbitals. We'll explore the electronic configuration of zinc and how it contributes to its chemical behavior.
Part 3: Zinc Orbital Diagram
3.1 Visualizing Electron Arrangement:
The zinc orbital diagram visually represents the distribution of electrons across different energy levels and sublevels. We'll delve into how the diagram is constructed and what each component signifies.
3.2 The Aufbau Principle:
The Aufbau principle guides the filling of atomic orbitals in a specific order. We'll explain how this principle applies to the construction of the zinc orbital diagram.
Part 4: Understanding the Diagram
4.1 Energy Levels and Sublevels:
In the zinc orbital diagram, energy levels and sublevels are represented through lines and boxes, respectively. We'll break down how these components indicate the electron arrangement.
4.2 Spin and Hund's Rule:
The arrows within the boxes of the zinc orbital diagram represent electron spin. We'll discuss the significance of electron spin and how Hund's rule influences the electron distribution.
Part 5: Implications in Chemistry
5.1 Chemical Properties of Zinc:
The electron arrangement depicted by the zinc orbital diagram plays a crucial role in determining the chemical properties of zinc. We'll explore how it affects bonding and reactivity.
5.2 Applications in Industry:
Zinc is widely used in various industries, from galvanizing metals to producing batteries. Understanding its electron arrangement helps predict its behavior in these applications.
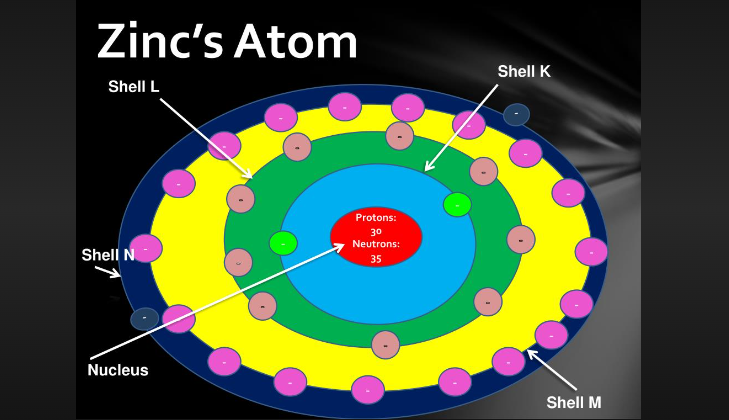
Zinc's Atom
The zinc orbital diagram serves as a fundamental tool in understanding the electron arrangement within the zinc atom. This diagram not only aids in visualizing the distribution of electrons but also contributes to our comprehension of zinc's chemical properties and behavior. By adhering to the principles of electron configuration, the zinc orbital diagram becomes an essential resource for both students of chemistry and professionals working with this versatile element.