What pH is Bleach? Understanding the pH Level of Bleach
Bleach is a commonly used household cleaning agent known for its powerful disinfecting and stain-removing properties. But have you ever wondered what the pH of bleach is? In this article, we will explore the pH scale, discuss the pH level of bleach, and understand its significance in cleaning and disinfection processes. Join us as we uncover the pH secrets of bleach.
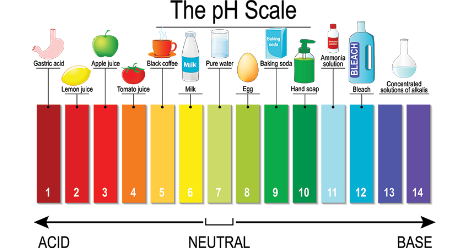
Acids and Base examples
I. The pH Scale and its Significance:
1. Definition of pH:
- The pH scale is a measurement system used to determine the acidity or alkalinity of a substance.
- It ranges from 0 to 14, where 0 represents extreme acidity, 7 indicates neutrality, and 14 signifies extreme alkalinity.
2. Importance of pH in Cleaning:
- pH plays a vital role in cleaning as it affects the efficiency and effectiveness of various cleaning agents.
- Different pH levels are suitable for specific cleaning tasks, ensuring optimal results.
II. pH Level of Bleach:
1. Alkaline Nature of Bleach:
- Bleach is an alkaline solution, meaning it has a pH level greater than 7.
- The pH level of bleach typically ranges from 11 to 13.
2. Sodium Hypochlorite Content:
- The primary active ingredient in household bleach is sodium hypochlorite (NaClO).
- Sodium hypochlorite contributes to the alkaline nature of bleach.
III. Significance of Bleach pH Level:
1. Disinfecting Properties:
- The high pH level of bleach contributes to its disinfecting properties.
- Alkaline environments created by bleach help eliminate various microorganisms, including bacteria and viruses.
2. Stain-Removing Abilities:
- The alkalinity of bleach assists in breaking down stains and removing colorants from fabrics and surfaces.
- It can effectively combat tough stains like those from coffee, tea, or grass.
3. Precautions and Safety Measures:
- The high pH level of bleach can be corrosive and irritating to the skin and eyes.
- Proper precautions, such as using gloves and ensuring adequate ventilation, are necessary when handling bleach.
Test PH- Changed color
The pH level of bleach falls within the alkaline range, typically ranging from 11 to 13. Its high pH contributes to its disinfecting properties and stain-removing abilities. Understanding the pH level of bleach is crucial for using it effectively and safely. Always take precautions when handling bleach due to its potential irritant properties. By considering the pH scale and its impact on cleaning agents like bleach, you can make informed decisions to achieve optimal cleaning and disinfection results in your household cleaning routines.