Understanding the Atomic Structure of Calcium: How Many Valence Electrons Does Calcium Have?
The atomic structure of an element plays a crucial role in determining its chemical properties and behavior. One essential aspect of an atom is its valence electrons, which are involved in chemical bonding and reactions. In this article, we will delve into the atomic structure of calcium, a fundamental element found in our everyday lives, and explore the number of valence electrons it possesses.
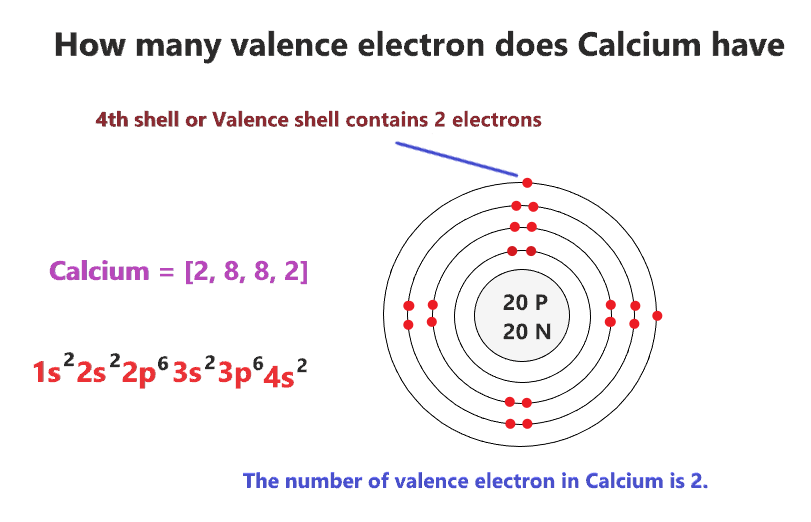
Atomic Structure of Calcium
1. The Basics of Atomic Structure
Before we delve into calcium's atomic structure, it's essential to understand the basic components of an atom. At the heart of an atom lies a dense nucleus, composed of protons and neutrons, while electrons orbit around the nucleus in energy levels or shells.
2. Calcium: An Overview
Calcium, with the symbol "Ca" in the periodic table, is an essential alkaline earth metal. It is the fifth most abundant element in the Earth's crust and serves various crucial functions in biological systems.
3. Electron Configuration of Calcium
To determine the number of valence electrons in calcium, we must first understand its electron configuration. The distribution of electrons in different energy levels is key to identifying the valence electrons.
4. Calcium's Electron Configuration
In this section, we will unravel the electron configuration of calcium step by step, providing insights into the arrangement of electrons in different energy levels around the nucleus.
5. Valence Electrons: Definition and Significance
Before we specifically discuss calcium's valence electrons, let's explore the concept of valence electrons and their significance in chemical interactions.
6. How to Determine the Number of Valence Electrons in an Element
Understanding the methodology to calculate the number of valence electrons in an element will allow us to accurately find the valence electrons of calcium.
7. Calcium's Valence Electrons
Now that we have the required knowledge, we will reveal the number of valence electrons calcium possesses, shedding light on its chemical reactivity and behavior.
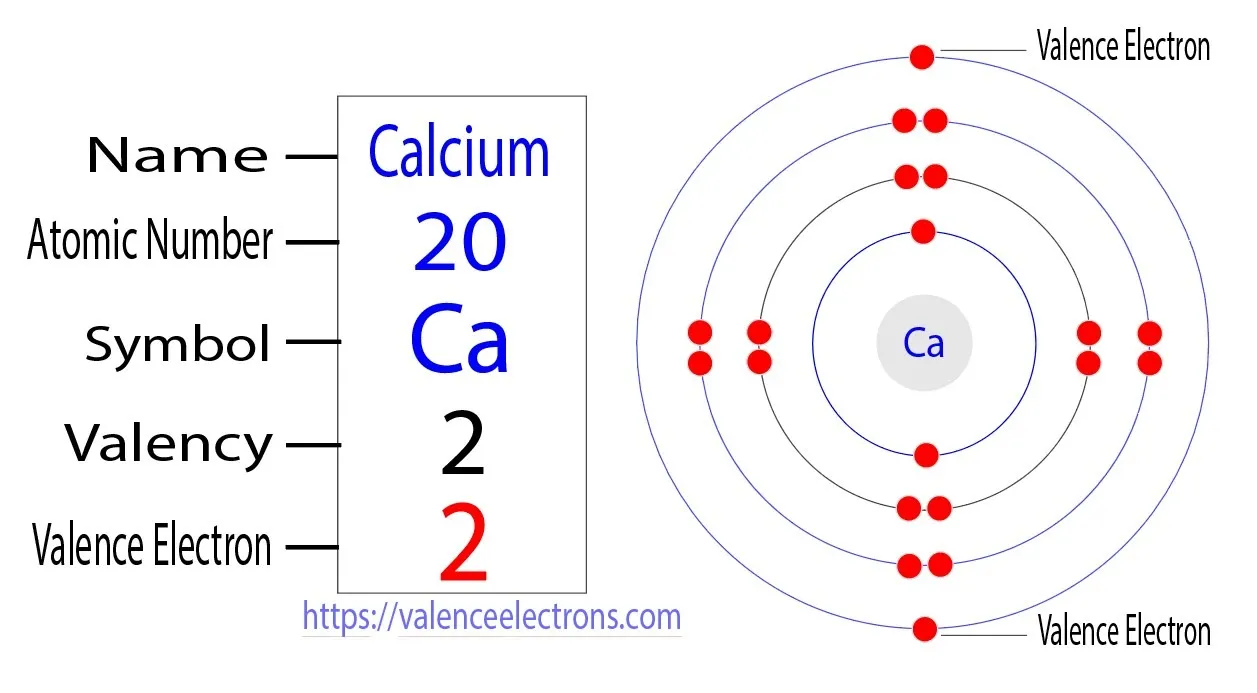
Atomic Structure of Calcium
8. The Role of Valence Electrons in Calcium's Chemical Properties
This section will elucidate the impact of valence electrons on calcium's chemical properties and its behavior when forming compounds.
9. Chemical Reactions of Calcium
With an understanding of calcium's valence electrons and its chemical properties, we can explore some typical chemical reactions that calcium undergoes.
10. Importance of Calcium's Valence Electrons in Biological Systems
Apart from its significance in chemical reactions, calcium's valence electrons are crucial for various biological processes. This section will highlight calcium's role in living organisms.
11. Conclusion
In conclusion, calcium is an indispensable element with important applications in various fields. Its atomic structure, particularly the valence electrons, plays a pivotal role in its chemical and biological behavior. Understanding the number and behavior of valence electrons in calcium enriches our comprehension of this essential element and its interactions in diverse environments.
Final Thoughts
By exploring the atomic structure of calcium and the number of valence electrons it possesses, we can better appreciate its role in our lives. Whether it's contributing to the formation of compounds or being essential for biological processes, calcium's valence electrons underpin its significance in the world of chemistry and biology. As we continue to advance our understanding of atomic structures, we unlock the door to countless possibilities and applications in science and technology.