Fluorine Orbital Notation: Unveiling the Secrets of Electrons' Arrangement
The study of atoms and their constituent particles has always fascinated scientists. One crucial aspect of understanding an atom's behavior lies in deciphering its electron configuration. In this article, we delve into the realm of fluorine, a highly reactive chemical element, and explore the intricacies of its orbital notation. Join us on this journey of unraveling the secrets hidden within fluorine's electron arrangement.
1. Understanding Fluorine:
Fluorine, with the atomic number 9, belongs to the halogen group of elements. It is the lightest and most electronegative halogen, exhibiting unique chemical properties.
Fluorine's electron configuration holds the key to comprehending its reactivity and its participation in various chemical reactions.
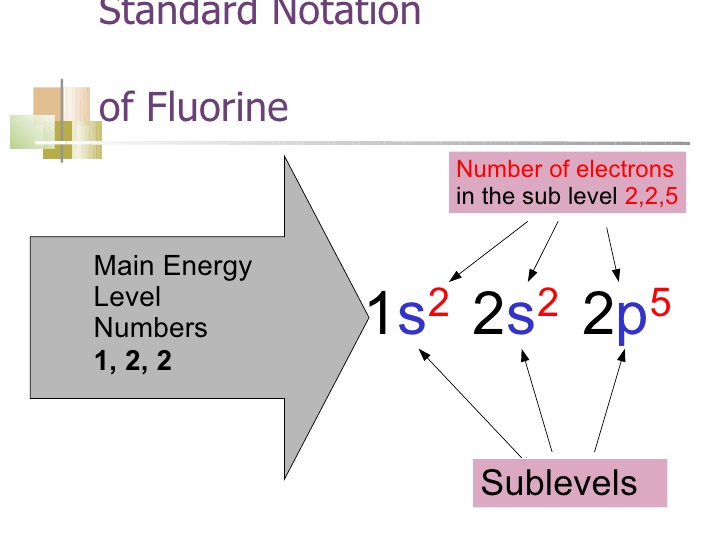
2. The Basics of Orbital Notation:
To understand fluorine's orbital notation, we must first grasp the fundamentals of electron arrangement. Electrons occupy specific energy levels and subshells within an atom, characterized by a set of quantum numbers.
The orbital notation provides a visual representation of these electrons' distribution in different orbitals.
3. Electron Configuration of Fluorine:
Fluorine has a total of nine electrons. To determine its electron configuration, we follow the Aufbau principle and Hund's rule.
By progressively filling the orbitals according to increasing energy levels, we arrive at the following electron configuration for fluorine: 1s² 2s² 2pâµ.

fluorine orbital notation
4. Breaking Down the Electron Configuration:
Let's break down the electron configuration of fluorine to gain a deeper understanding of its arrangement. The 1s² portion represents the first energy level, consisting of a single s orbital holding two electrons.
Moving on to the second energy level, we have the 2s² portion, indicating a pair of electrons in the s orbital. Lastly, the 2pâµ segment reveals five electrons distributed across the three 2p orbitals.
5. Orbital Diagram of Fluorine:
To visualize fluorine's electron distribution, we can employ an orbital diagram. Each orbital is represented by a box, and electrons are depicted as arrows within the boxes.
The orbital diagram for fluorine showcases two filled boxes in the 1s orbital, two filled boxes in the 2s orbital, and five arrows in the three 2p orbitals.
fluorine orbital notation
6. Significance of Electron Configuration:
Fluorine's electron configuration, 1s² 2s² 2pâµ, plays a vital role in explaining its reactivity.
The partially filled 2p orbitals in fluorine's valence shell make it highly reactive, as it seeks to achieve a stable, fully-filled electron configuration through bonding with other elements.
7. Chemical Properties of Fluorine:
The electron configuration of fluorine influences its chemical properties significantly. As a halogen, fluorine tends to gain an electron to complete its octet, making it a potent oxidizing agent.
Its electronegativity and small atomic size allow it to form strong bonds and participate in various chemical reactions, showcasing its high reactivity.
8. Applications and Implications:
Fluorine's unique electron configuration has several practical applications. Its compounds are used in the production of numerous chemicals, including refrigerants, polymers, and pharmaceuticals.
Moreover, fluorine plays a crucial role in industries such as electronics, dentistry, and water treatment.

fluorine orbital notation
9. Conclusion:
Understanding the orbital notation of fluorine provides valuable insights into its electron arrangement, leading to a deeper comprehension of its reactivity and chemical properties.
Through the exploration of fluorine's electron configuration, we have uncovered the secrets behind its unique behavior.
By continually unraveling the mysteries of atomic structure, scientists continue to expand our knowledge of the building blocks of the universe.