Valence Electrons in Germanium: Understanding the Electron Configuration
Germanium is an important element in the periodic table, and its electron configuration plays a crucial role in determining its chemical properties. In this article, we will explore the concept of valence electrons in germanium and delve into its electron configuration. Understanding the valence electrons in germanium is essential for comprehending its chemical behavior and its role in various applications.

Valence electrons in Gr
1. Section 1: Introduction to Germanium
1.1 Overview of Germanium
Germanium is a chemical element with the atomic number 32 and the symbol Ge. It is a hard, brittle, grayish-white metalloid that is commonly found in minerals such as germanite and argyrodite. Germanium has several unique properties that make it valuable in various applications, including electronics and optics.
1.2 Importance of Valence Electrons
Valence electrons are the outermost electrons in an atom that participate in chemical reactions and bond formation. They determine an element's chemical reactivity and behavior. Understanding the valence electrons in germanium is essential for predicting its bonding patterns and reactions with other elements.
2. Section 2: Electron Configuration of Germanium
2.1 Understanding Electron Configuration
Electron configuration refers to the arrangement of electrons in the energy levels and orbitals of an atom. It is represented using a notation that indicates the distribution of electrons among the different orbitals.
2.2 Electron Configuration of Germanium
The electron configuration of germanium is [Ar] 3d10 4s2 4p2. This configuration indicates that germanium has two electrons in its 4s orbital, two electrons in its 4p orbital, and ten electrons in its 3d orbital. The outermost energy level, known as the valence shell, is the 4th energy level.
3. Section 3: Valence Electrons in Germanium
3.1 Determining Valence Electrons
Valence electrons are located in the outermost energy level of an atom. For germanium, the valence electrons are found in the 4th energy level (4s and 4p orbitals). To determine the number of valence electrons in germanium, we count the electrons in these orbitals.
3.2 Number of Valence Electrons in Germanium
Germanium has four valence electrons. These are the two electrons in the 4s orbital and the two electrons in the 4p orbital. The presence of four valence electrons makes germanium an element that can form stable covalent bonds with other elements.
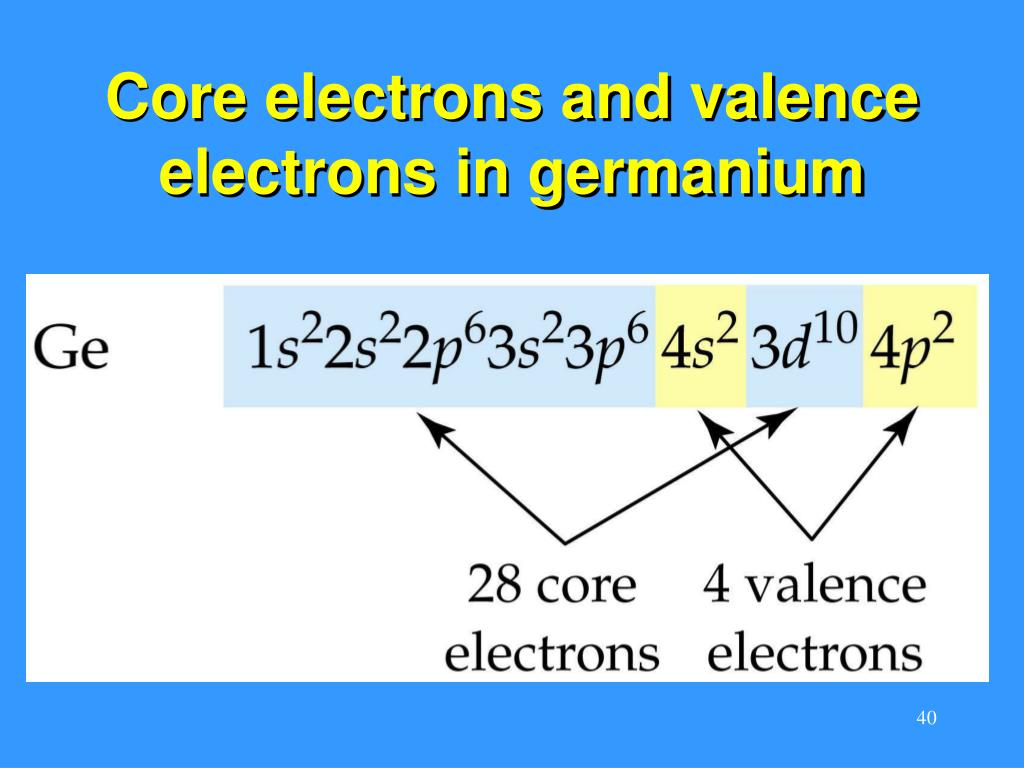
Core electrons and valence electrons in fermanium
Valence electrons play a crucial role in determining the chemical properties and behavior of germanium. By understanding the electron configuration of germanium and the number of valence electrons it possesses, we gain insights into its reactivity, bonding behavior, and its applications in various fields. Whether you are a student, a researcher, or simply curious about the element germanium, understanding its valence electrons provides valuable knowledge about this versatile element's chemical nature.