The Elements Least Likely to Form Bonds: Group 15, 16, 17, or 18?
When it comes to chemical bonding, certain elements have unique properties that make them less likely to form bonds with other elements. In this article, we will explore the elements that are least likely to form bonds and identify which group they belong to. The focus will be on Group 15, 16, 17, and 18 elements and their characteristics regarding chemical bonding.
Periodic table
1. Group 15 Elements: Nitrogen Family
The elements in Group 15 of the periodic table include nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi).
These elements are known as the nitrogen family. Group 15 elements have five valence electrons, making them highly stable and less likely to form bonds.
Nitrogen, for example, has a stable electron configuration with three pairs of electrons in its valence shell, making it energetically unfavorable to gain or lose electrons.
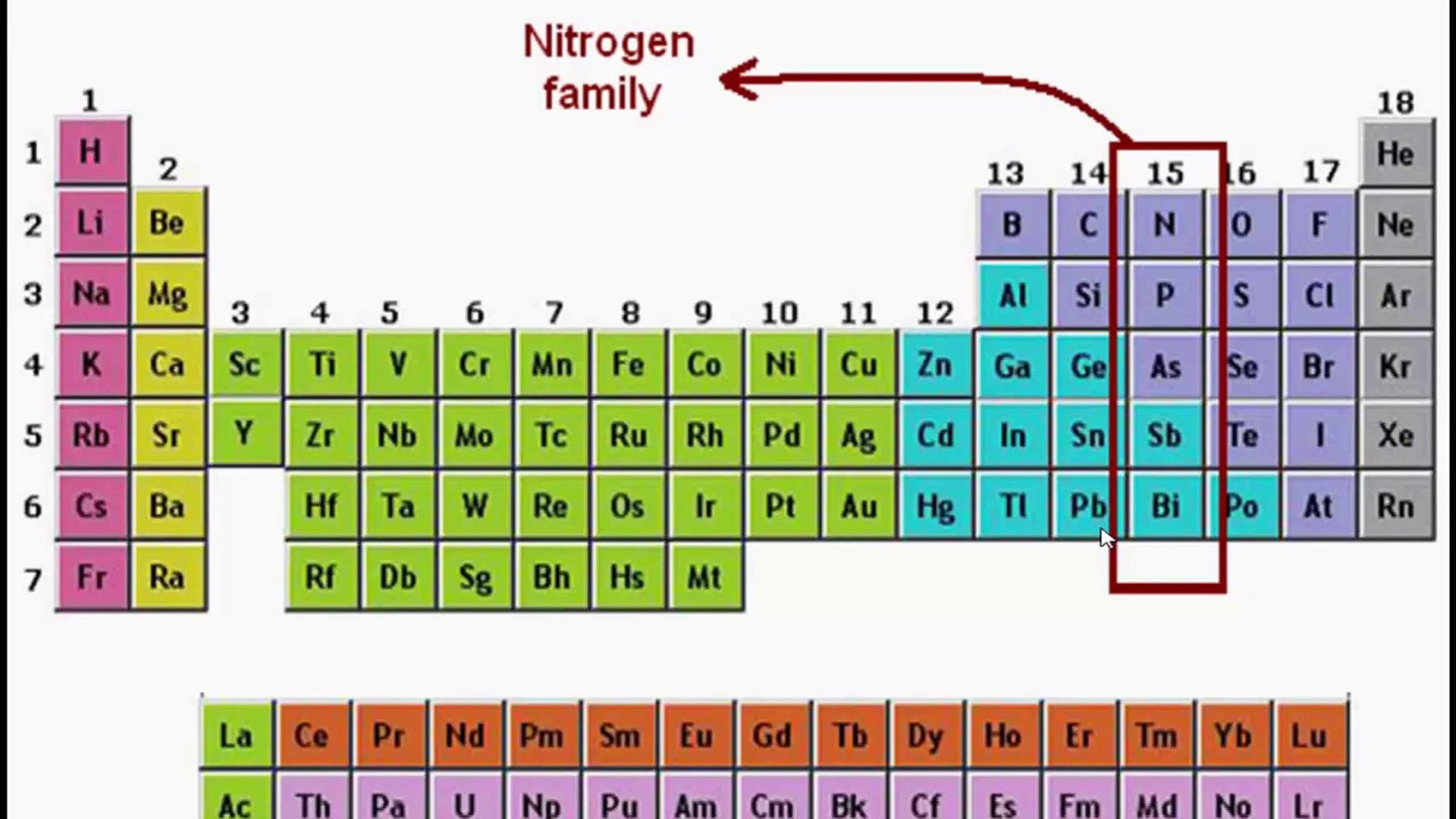
Nitrogen Family
2. Group 16 Elements: Oxygen Family
The elements in Group 16, also known as the oxygen family, include oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po).
Group 16 elements have six valence electrons, resulting in a stable electron configuration.
Oxygen, for instance, achieves stability by forming a double bond with another oxygen atom, resulting in the formation of O2, which makes it less likely to form additional bonds.
3. Group 17 Elements: Halogens
The elements in Group 17 are known as halogens and include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Halogens have seven valence electrons, making them highly reactive. However, despite their reactivity, they do not readily form covalent bonds with other halogens.
For example, chlorine gas (Cl2) consists of two chlorine atoms bonded together through a covalent bond, but it does not easily form bonds with additional chlorine atoms.
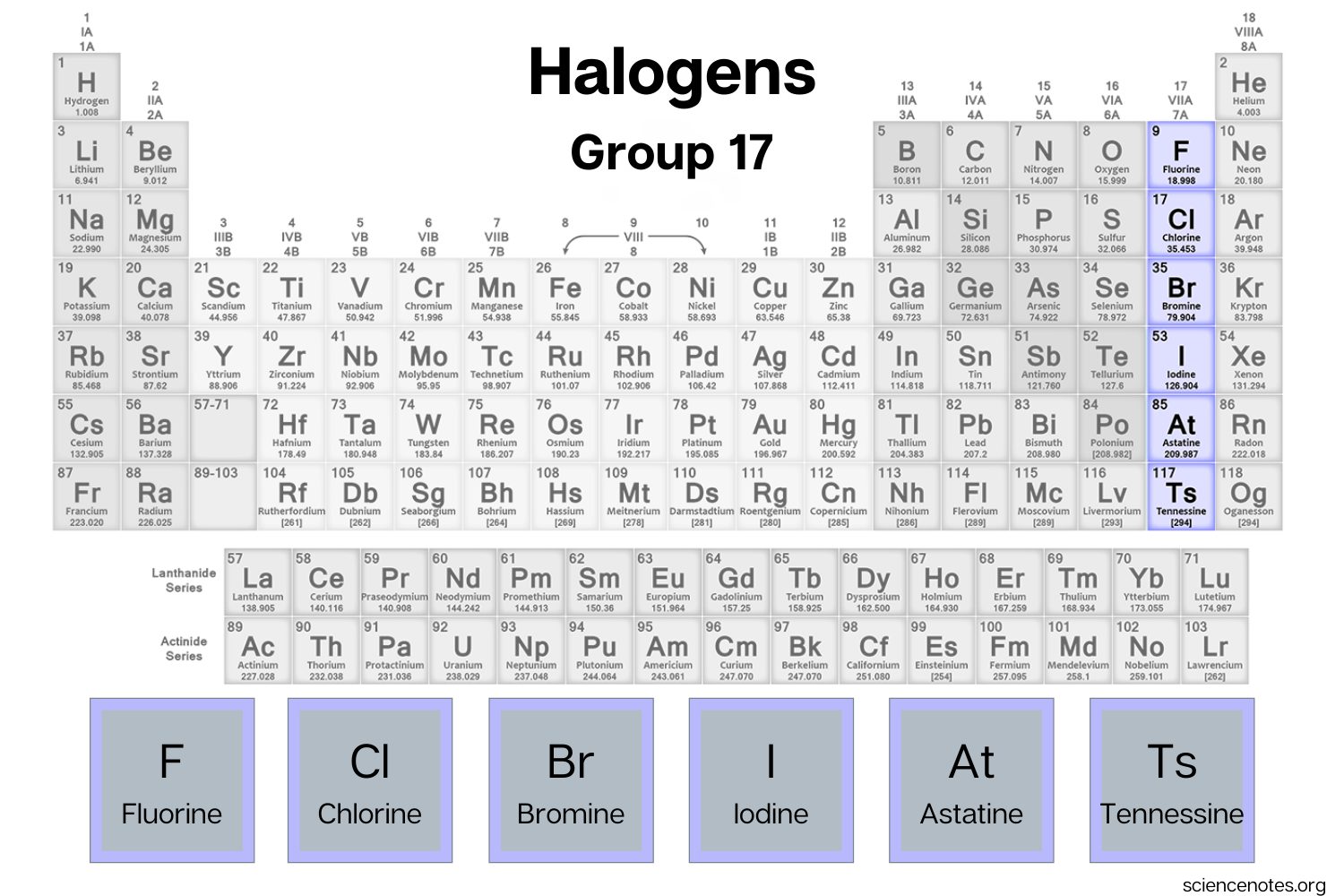
Halogen Elements
4. Group 18 Elements: Noble Gases
Group 18 elements, also referred to as noble gases, include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Noble gases have full valence electron shells, making them extremely stable and unreactive.
Their electron configurations make it highly unlikely for them to form bonds with other elements.
These elements were historically considered inert due to their lack of reactivity, earning them the name "noble gases."
Noble Gases
In conclusion, the elements least likely to form bonds are found in Group 15, 16, 17, and 18 of the periodic table. Group 15 elements, including nitrogen and phosphorus, have stable electron configurations that make them less likely to form bonds.
Group 16 elements, such as oxygen and sulfur, also possess stable configurations due to their valence electron count. Halogens in Group 17 are highly reactive but do not readily form covalent bonds with each other. Lastly, noble gases in Group 18, including helium and xenon, have full valence electron shells, making them extremely stable and unreactive.
Understanding the properties of these elements and their tendencies to form bonds is essential for studying chemical reactions and understanding the behavior of different elements within the periodic table. By recognizing the patterns and trends in bonding, scientists can further expand their knowledge of the elements and their interactions.