Which Species Is a Conjugate Acid of OH? Exploring Acid-Base Reactions
In the realm of chemistry, acid-base reactions play a fundamental role in understanding the behavior and properties of substances. These reactions involve the transfer of protons (H+) between species, resulting in the formation of conjugate acid-base pairs. In this article, we will explore the identity of the species that acts as a conjugate acid of OH (hydroxide ion). Understanding this relationship is crucial in comprehending acid-base equilibrium and the concept of conjugate acid-base pairs. So, let's delve into the world of acid-base chemistry and unveil the mystery of the conjugate acid of OH.
1. Acid-Base Reactions and Conjugate Pairs
Acid-Base
Acid-base reactions involve the transfer of protons (H+) from one species, known as the acid, to another, known as the base. The acid donates a proton, while the base accepts it.
In these reactions, conjugate acid-base pairs are formed, where the acid in one pair becomes the base in the other, and vice versa.
2. The OH Ion as a Base
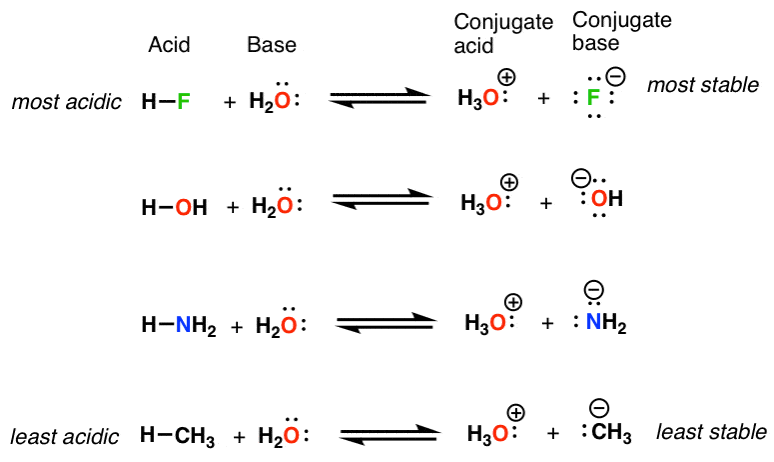
OH Ion as a Base
The OH ion, or hydroxide ion, is a base due to its ability to accept a proton (H+).
It acts as a base in many acid-base reactions, accepting a proton from an acid to form water (H2O).
3. Definition of Conjugate Acid
In acid-base reactions, the species formed after the base accepts a proton is called the conjugate acid. It is the result of the base gaining a proton.
4. Identifying the Conjugate Acid of OH
To determine the conjugate acid of OH, we need to examine the acid-base reaction involving OH.
When OH accepts a proton, it forms water. Therefore, the conjugate acid of OH is water (H2O).
5. Water as a Conjugate Acid
In the context of acid-base reactions involving OH, water acts as a conjugate acid.
When OH accepts a proton, it gains the proton and becomes a hydronium ion (H3O+), which is a stronger acid than water.
6. Acid-Base Equilibrium and Proton Transfer
Understanding the concept of conjugate acids and bases is essential in acid-base equilibrium.
In a reversible reaction, the forward reaction involves the transfer of a proton from the acid to the base, while the reverse reaction involves the transfer of a proton from the conjugate acid to the conjugate base.
This equilibrium allows for the establishment of acid-base balance.
7. Examples of Acid-Base Reactions Involving OH
Acid-base reactions involving OH occur in various chemical processes. Some examples include:
Neutralization Reactions: In reactions between acids and bases, OH acts as the base, accepting a proton from the acid to form water.
Acid Dissociation: Some acids can dissociate in water, producing H+ ions and their respective conjugate bases.
For example, the dissociation of acetic acid (CH3COOH) produces CH3COO- as the conjugate base and H3O+ as the conjugate acid.
8. Importance of Acid-Base Reactions
Acid-base reactions are fundamental in various scientific disciplines, including chemistry, biology, and environmental science.
They help explain chemical behavior, buffer systems, pH regulation, and the functioning of biological systems.
In conclusion, the species that acts as a conjugate acid of OH (hydroxide ion) is water (H2O). In acid-base reactions, OH functions as a base, accepting a proton to form water. Water, in turn, can act as a conjugate acid when it gains a proton, forming the hydronium ion (H3O+). Understanding the concept of conjugate acid-base pairs is crucial in comprehending acid-base equilibrium and the proton transfer that occurs in chemical reactions. By unraveling the mysteries of acid-base chemistry, we gain valuable insights into the behavior and properties of substances, contributing to advancements in various scientific fields. So, let us continue to explore and appreciate the fascinating world of acid-base reactions and their significance in understanding the nature of matter.