Water Boils Quicker in Denver, Colorado (1 Mile in Altitude) than at the Beach
Water boiling is a fundamental process that occurs when the temperature of a liquid reaches its boiling point, causing it to change from a liquid to a gas phase. However, did you know that water boils quicker in Denver, Colorado, which is situated at a high altitude of approximately one mile, compared to the beach? This phenomenon is an intriguing aspect of physics and has practical implications for various activities, such as cooking and brewing. In this article, we will explore the reasons behind this occurrence and delve into the science behind the quicker boiling process in Denver.

Boiling Water
Altitude and Atmospheric Pressure
To understand why water boils quicker in Denver, we must first grasp the relationship between altitude and atmospheric pressure.
Atmospheric pressure refers to the force exerted by the Earth's atmosphere on a given area. Generally, the higher the altitude, the lower the atmospheric pressure.
This decrease in pressure occurs because the density of the atmosphere decreases as we move further away from the Earth's surface.
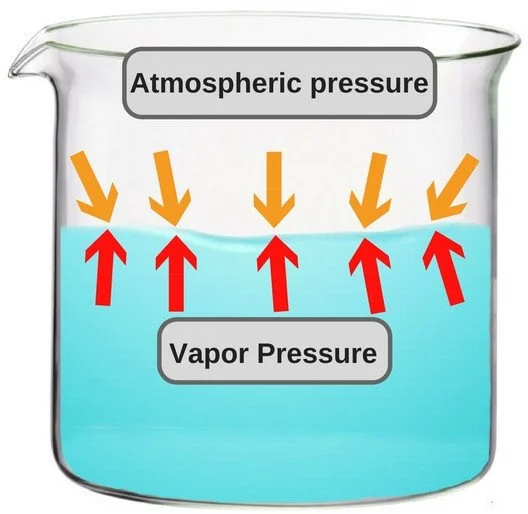
Vapor and atmospheric pressure
Boiling Point and Vapor Pressure
The boiling point of a substance is the temperature at which its vapor pressure equals the external pressure applied to it.
In simpler terms, it is the temperature at which a liquid turns into a gas within the liquid itself.
As atmospheric pressure decreases with increasing altitude, the boiling point of water also decreases.
The Science behind Quicker Boiling in Denver
1. Lower Boiling Point: Due to the lower atmospheric pressure in Denver, the boiling point of water decreases. At sea level, water typically boils at 100 degrees Celsius (212 degrees Fahrenheit), but in Denver, it boils at approximately 95 degrees Celsius (203 degrees Fahrenheit). The lower boiling point results in quicker boiling times.
2. Decreased Heat Transfer: Another factor contributing to quicker boiling in Denver is the reduced heat transfer. As water boils, it undergoes a phase change from liquid to gas. During this process, heat energy is absorbed to convert the liquid molecules into gaseous molecules. At higher altitudes, where the atmospheric pressure is lower, the heat transfer rate decreases, resulting in faster boiling.
3. Increased Evaporation: The lower atmospheric pressure in Denver also enhances the rate of evaporation. As water reaches its boiling point, the reduced pressure allows water molecules to escape more easily, leading to increased evaporation. This increased evaporation further accelerates the boiling process.
Practical Implications
The quicker boiling time in Denver holds practical implications for several activities:
1. Cooking: In Denver, where water boils at a lower temperature, cooking times for certain foods may need adjustment. Recipes that rely on precise boiling times, such as pasta or boiled eggs, require shorter cooking durations in Denver compared to coastal areas.
2. Brewing: Beer brewing enthusiasts in Denver can take advantage of the quicker boiling time to reduce the overall brewing process. This can be beneficial for homebrewers and commercial breweries alike, allowing for increased efficiency.
Water boils quicker in Denver, Colorado, situated at a high altitude of approximately one mile, due to the lower atmospheric pressure. The decrease in pressure leads to a lower boiling point for water, resulting in faster boiling times. Understanding this phenomenon has practical implications for cooking, brewing, and other activities that rely on precise boiling times. Whether you find yourself at the beach or in Denver, appreciating the science behind this natural phenomenon can deepen your understanding of the world around us.