Which Substances are Least Likely to Completely Dissolve in Water?
When it comes to the solubility of substances in water, not all materials are created equal. Some readily dissolve, forming a homogeneous mixture, while others exhibit limited or no solubility, remaining largely unaffected by water molecules. In this article, we explore the concept of solubility and investigate which substances are least likely to completely dissolve in water. Join us as we delve into the characteristics of various substances and understand why they resist dissolution in the aqueous medium.
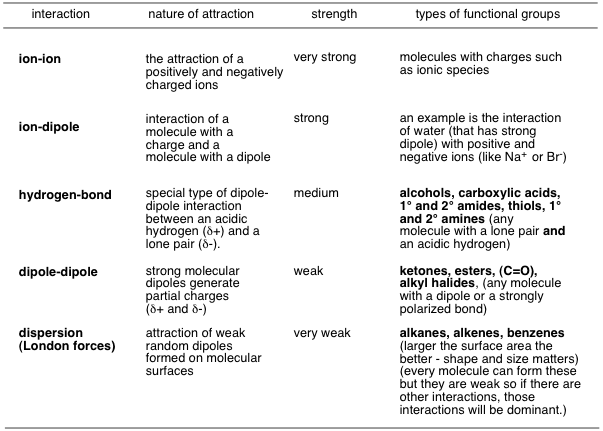
Compound disolve in water table
1. Understanding Solubility
- Definition of Solubility: Solubility refers to the ability of a substance to dissolve in a solvent, such as water, to form a homogeneous solution.
- Factors Affecting Solubility: Several factors influence solubility, including temperature, pressure, and the chemical nature of the solute and solvent.
2. Polar vs. Non-Polar Substances
- Polar Substances: Polar substances possess an uneven distribution of electron density, resulting in partial positive and negative charges. These substances often dissolve well in water due to its polar nature.
- Non-Polar Substances: Non-polar substances have an even distribution of electron density and lack significant partial charges. As a result, they exhibit limited solubility or are insoluble in water.
3. Substances with Limited Solubility
- Fats and Oils: Fats and oils are hydrophobic substances composed of non-polar molecules. Due to their non-polar nature, they are unable to form favorable interactions with water molecules, making them insoluble in water.
- Hydrophobic Compounds: Many organic compounds, such as hydrocarbons, do not dissolve well in water due to their non-polar nature.
- Insoluble Salts: Certain salts, such as silver chloride (AgCl) and lead(II) sulfate (PbSO4), have limited solubility in water and tend to form precipitates rather than dissolving completely.
4. Exceptions and Partial Solubility
- Slightly Soluble Salts: Some salts exhibit partial solubility in water, meaning they dissolve to a limited extent. Examples include calcium sulfate (CaSO4) and barium sulfate (BaSO4).
- Amphipathic Molecules: Amphipathic molecules have both polar and non-polar regions. While the polar part can interact with water, the non-polar region resists dissolution. Examples include phospholipids found in cell membranes.
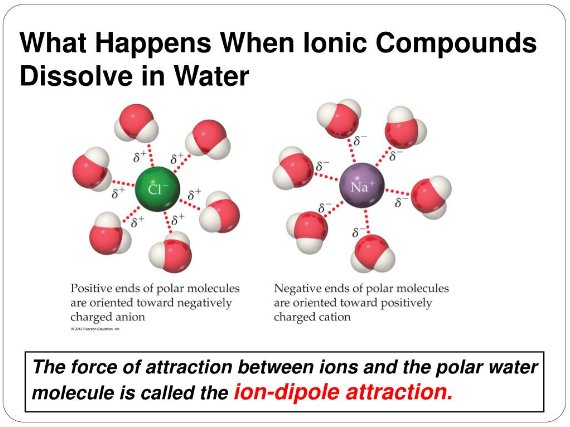
What happens when lonic compounds dissolve in water
The solubility of substances in water varies depending on their chemical nature. While polar substances readily dissolve in water, non-polar substances, such as fats, oils, and hydrophobic compounds, exhibit limited solubility or are insoluble. Additionally, some salts have limited solubility and may form precipitates rather than dissolving completely. It is important to note that there are exceptions and variations in solubility, depending on the specific substance and conditions. Understanding the solubility properties of different materials contributes to our knowledge of chemical interactions and has practical applications in various fields.