Why Are Metals Shiny: A Comprehensive Explanation
Metals have fascinated humanity for centuries due to their unique properties and applications in various fields. One of the most striking characteristics of metals is their shiny appearance. In this article, we delve into the reasons behind the shininess of metals, exploring the underlying scientific principles and phenomena that contribute to this captivating feature.
1. Electromagnetic Interaction
The primary reason metals exhibit a shiny appearance lies in their ability to interact with light through the process of electromagnetic interaction. When light falls on the surface of a metal, it interacts with the free electrons present in the metal's structure. This interaction leads to the reflection and absorption of light, resulting in the observed shine.
Electromagnetic Interaction
2. Metallic Bonding
To understand why metals possess free electrons, we need to delve into the concept of metallic bonding. Metals are made up of a lattice structure consisting of positively charged metal ions immersed in a "sea" of delocalized electrons. This unique arrangement allows the free movement of electrons within the metal, which is crucial for their shiny nature.
3. Reflectivity
One key aspect contributing to the shininess of metals is their high reflectivity. Due to the presence of free electrons, incident light is efficiently reflected by the metal surface. This reflection occurs at the atomic level, with light waves bouncing off the surface, preserving the wave's energy and frequency. Consequently, metals can appear highly reflective and shiny to the human eye.
4. Absorption and Scattering
While metals are excellent reflectors, they also exhibit some absorption and scattering of light. When light interacts with the metal's surface, a portion of it is absorbed by the metal's electrons, leading to the excitation of these electrons to higher energy levels. The absorbed light energy can subsequently be re-emitted as scattered light, contributing to the overall shininess of the metal.
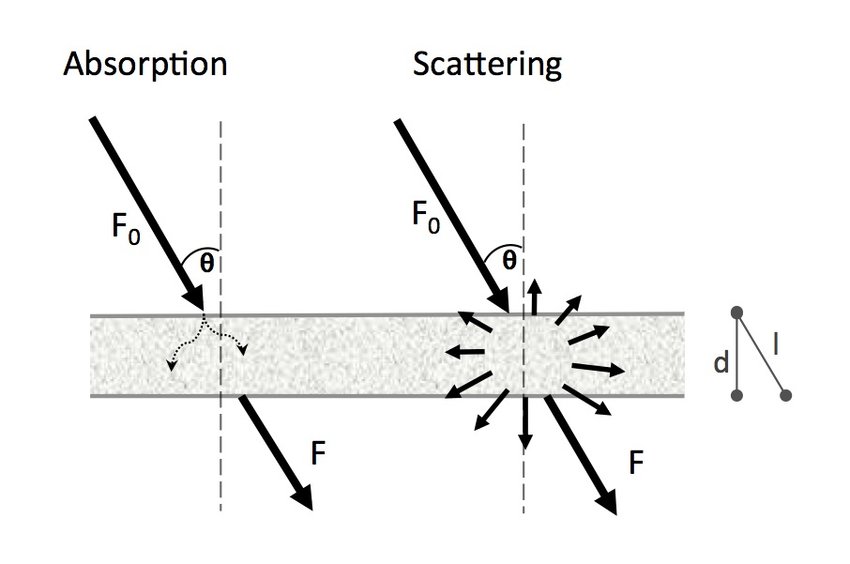
Absorption and Scattering
5. Smooth Surface
The smoothness of a metal surface plays a significant role in its shiny appearance. When a metal is polished, its surface becomes exceptionally smooth, minimizing surface imperfections and irregularities. This smoothness allows incident light to reflect off the surface more uniformly, resulting in a brighter and more consistent shine.
6. Molecular Structure
The molecular structure of metals also contributes to their shininess. Metals tend to have a closely packed arrangement of atoms, which promotes efficient reflection and scattering of light. This regular arrangement prevents light from being absorbed or diffused significantly, ensuring that most incident light is reflected, enhancing the metal's overall shine.
7. Optical Properties
The optical properties of metals, such as their refractive index and the wavelength dependence of their reflectivity, further influence their shiny appearance. Metals typically have a high refractive index, which affects the speed of light passing through them. This, in turn, affects the angle at which light is reflected, leading to enhanced shininess.
8. Surface Oxidation
In some cases, the presence of a thin layer of oxide on the metal's surface can impact its shininess. Oxidation can occur due to exposure to air or moisture, leading to the formation of a thin oxide layer. While this layer may slightly diminish the metal's shine, it can also create unique visual effects, such as a patina, which is highly valued in certain applications.
The shininess of metals can be attributed to a combination of factors, including electromagnetic interaction, metallic bonding, reflectivity, absorption and scattering, surface smoothness, molecular structure, optical properties, and even surface oxidation. Understanding these underlying principles provides valuable insights into why metals possess their distinctive shiny appearance. The ability to harness and manipulate these properties has enabled humans to utilize metals for a wide range of applications, from decorative purposes to technological advancements.