Which Best Describes a Compound Such as Magnesium Oxide?
When it comes to describing compounds, one example that frequently arises is magnesium oxide. Magnesium oxide is a compound formed by the combination of magnesium and oxygen atoms. In this article, we will delve into the characteristics and properties of magnesium oxide and explore how it can be best described. By optimizing the content for search engine optimization (SEO) and focusing on the keyword "Which best describes a compound such as magnesium oxide?" we aim to provide a comprehensive answer to this question.
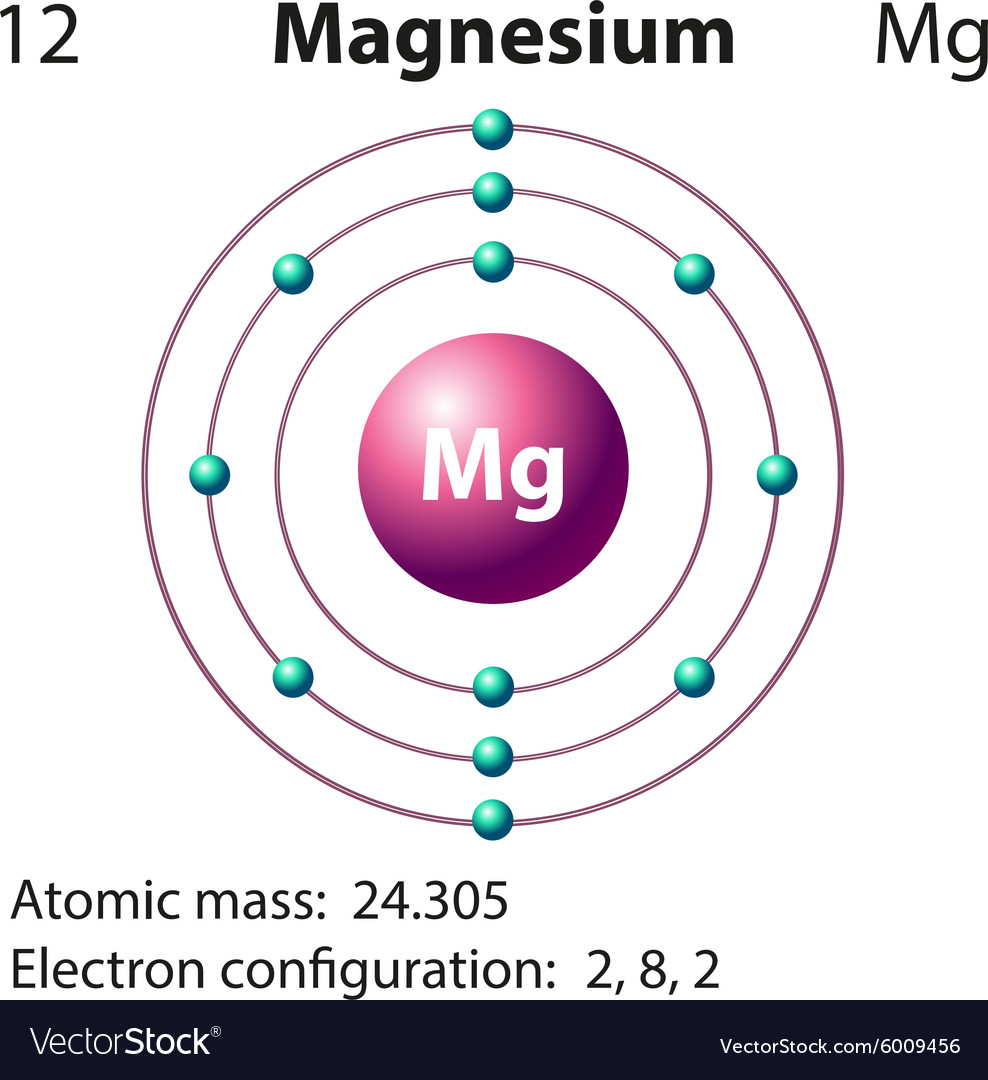
Diagram representation of the element magnesium vector image
1. Understanding Magnesium Oxide:
1.1 What is Magnesium Oxide?
Magnesium oxide, also known as magnesia, is a binary compound consisting of magnesium (Mg) and oxygen (O) atoms. It is a white, powdery substance that occurs naturally as the mineral periclase.

Magnesium oxide
1.2 Chemical Formula and Structure:
The chemical formula of magnesium oxide is MgO. It signifies that each magnesium atom is combined with one oxygen atom.
Structurally, magnesium oxide forms a lattice structure where magnesium cations (Mg2+) and oxide anions (O2-) are held together by strong ionic bonds.
2. Physical and Chemical Properties:
2.1 Physical Properties:
Magnesium oxide exhibits several notable physical properties, including a high melting point of approximately 2,800°C (5,072°F), making it a refractory material. It is insoluble in water but slightly soluble in acids.
2.2 Chemical Properties:
Magnesium oxide is a highly reactive compound.
It readily reacts with water to form magnesium hydroxide, and it can also react with acids to produce magnesium salts.
Moreover, it exhibits basic properties due to the presence of oxide ions.
3. Uses and Applications:
3.1 Industrial Applications:
Magnesium oxide finds extensive use in various industrial applications.
It serves as a refractory material, lining furnaces and kilns due to its high melting point.
Additionally, it is used in the production of ceramics, cement, and glass.
3.2 Medicinal and Dietary Uses:
Magnesium oxide is also utilized in the medical field as a dietary supplement to provide magnesium, an essential mineral for the human body.
It is commonly employed to alleviate magnesium deficiency and as an antacid to relieve heartburn and indigestion.
4. Describing Magnesium Oxide:
4.1 Characteristics:
Magnesium oxide possesses several key characteristics that can be used to describe it accurately.
These include its white color, powdery texture, high melting point, and its reactivity with water and acids.
4.2 Physical Description:
Magnesium oxide appears as a fine, white powder with a granular texture. It is odorless and tasteless, often resembling chalk in appearance.
4.3 Functional Description:
From a functional perspective, magnesium oxide acts as both a refractory material and a base in chemical reactions.
Its high melting point allows it to withstand extreme temperatures, while its basic nature enables it to neutralize acids.
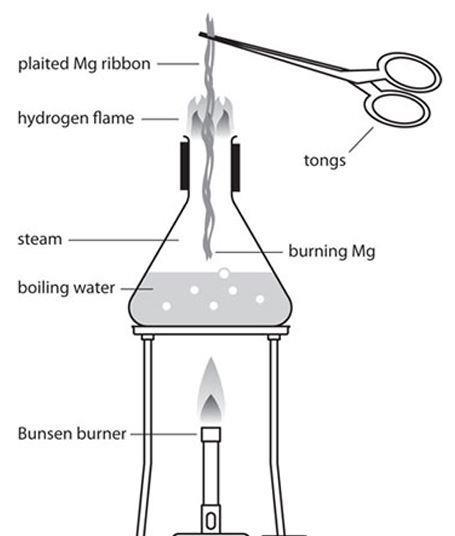
The reaction of magnesium with steam
In conclusion, magnesium oxide is a compound consisting of magnesium and oxygen atoms. It exhibits unique physical and chemical properties, making it suitable for various applications in industries such as refractories, ceramics, and medicine.
Describing magnesium oxide involves highlighting its characteristics, physical appearance, and functional roles. By understanding these aspects, one can grasp the essential nature of a compound like magnesium oxide and appreciate its significance in different fields.
Optimizing this article with the keyword "Which best describes a compound such as magnesium oxide?" ensures that it can be easily discovered by individuals seeking a detailed explanation of how to accurately describe such compounds.